|
|
|
|
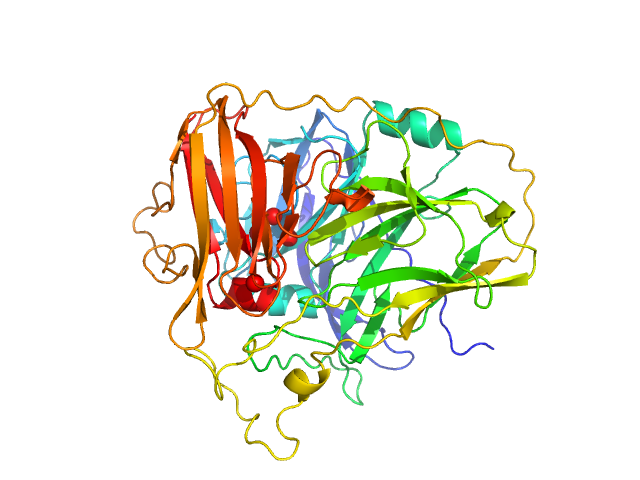
|
| Sample: |
McoA evolved variant 2F4 (Periplasmic cell division protein (SufI)) monomer, 55 kDa Aquifex aeolicus VF5 protein
|
| Buffer: |
50 mM Tris-HCl, 150 mM NaCl, 2 mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Sep 25
|
Distal Mutations Shape Substrate-Binding Sites during Evolution of a Metallo-Oxidase into a Laccase
ACS Catalysis :5022-5035 (2022)
...Borges P, Núñez-Franco R, Lucas M, Frazão C, Monza E, Masgrau L, Cordeiro T, Martins L
|
| RgGuinier |
2.3 |
nm |
| Dmax |
6.8 |
nm |
| VolumePorod |
78 |
nm3 |
|