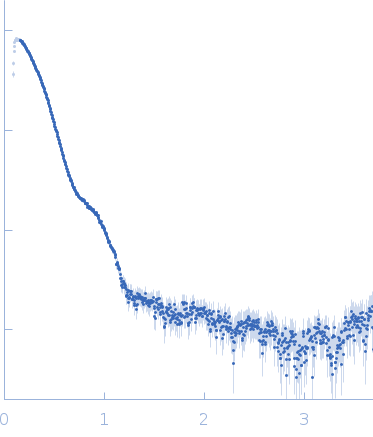
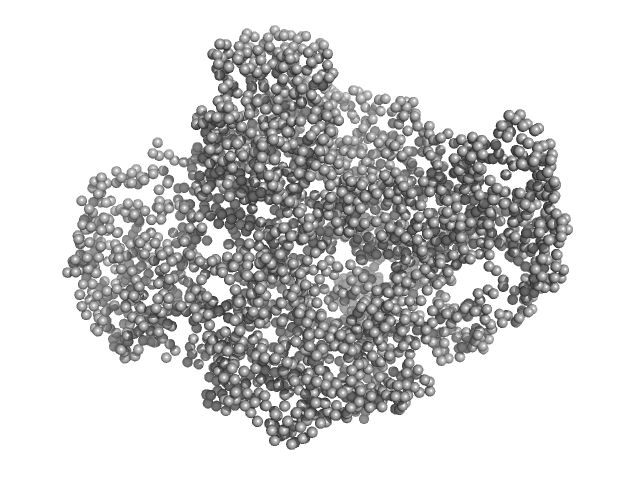
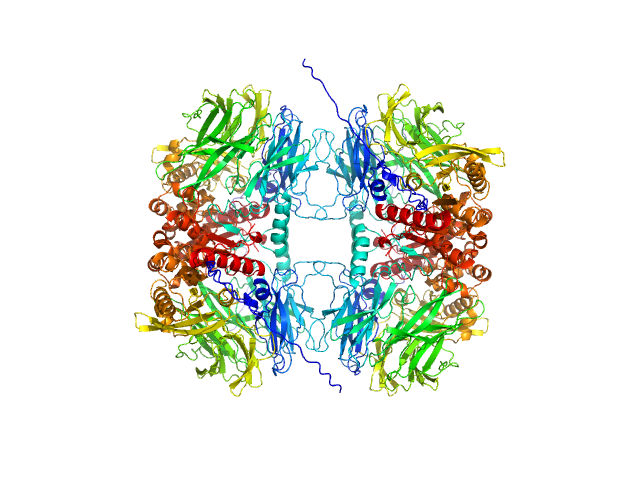
| MWexperimental | 318 | kDa |
| MWexpected | 304 | kDa |
| VPorod | 563 | nm3 |
|
log I(s)
7.64×100
7.64×10-1
7.64×10-2
7.64×10-3
|
 s, nm-1
s, nm-1
|
|
|
|

|
|
|
|
|
|
Synchrotron SAXS data from solutions of S9 peptidase form Mycobacterium phlei in 10 mM Tris-HCl, 135 mM NaCl, pH 8 were collected on the BL-18 beam line at INDUS-2 (Indore, India) using a MAR 345 Image Plate detector at a sample-detector distance of 2.2 m and at a wavelength of λ = 0.1033 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 4.00 mg/ml was measured at 25°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
X-ray Exposure time = UNKNOWN. Number of frames = UNKNOWN |
|
|||||||||||||||||||||||||||