|
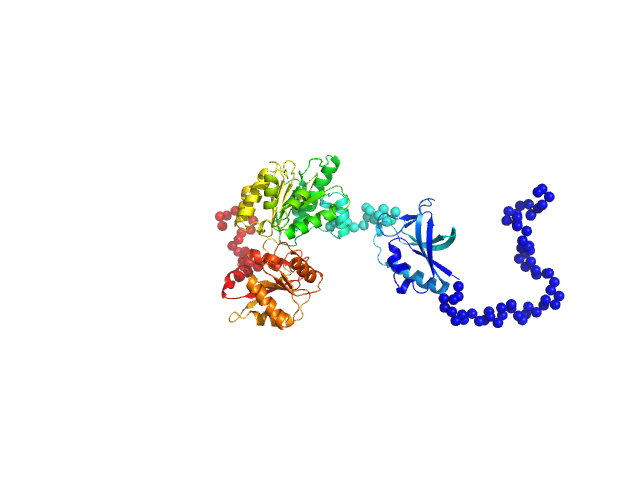
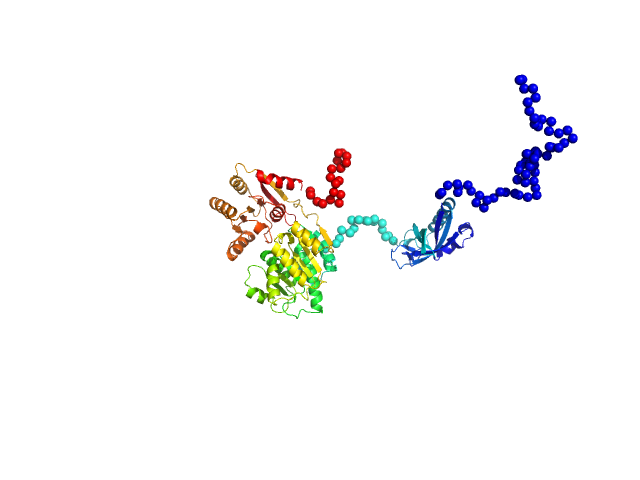
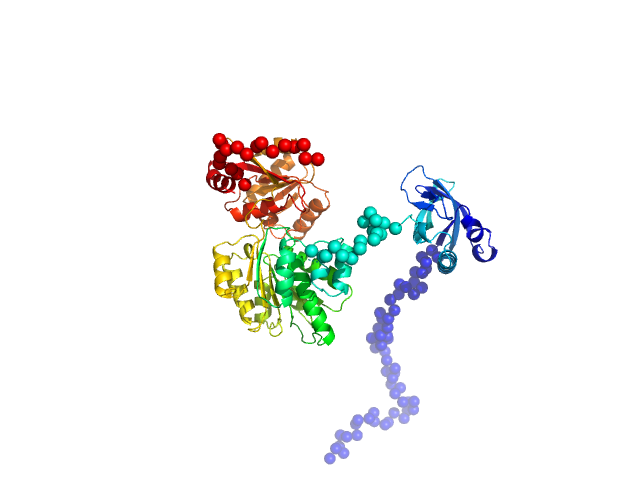
SAXS data from solutions of RnaH in 20 mM HEPES, 300 mM NaCl, pH 8 were collected on a Xenocs Xeuss 2.0 Q-Xoom instrument at the Center for Structural Studies, Heinrich-Heine-University (Düsseldorf, Germany) using a Pilatus3 R 300K detector at a sample-detector distance of 0.6 m and at a wavelength of λ = 0.154 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 1.2 and 9 mg/ml were measured at 10°C. 18 successive 600 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
RNA helicase (RnaH)
(crTPpart2_RnaH +RnaH)
|
| Mol. type |
|
Protein |
| Organism |
|
Paulinella chromatophora |
| Olig. state |
|
Monomer |
| Mon. MW |
|
63.5 kDa |
| Sequence |
|
FASTA |
| |
|
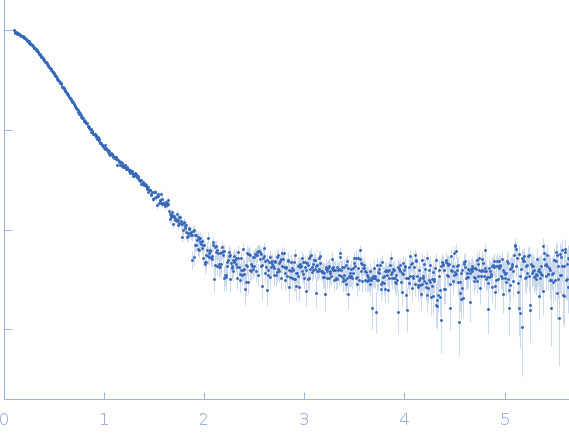 s, nm-1
s, nm-1
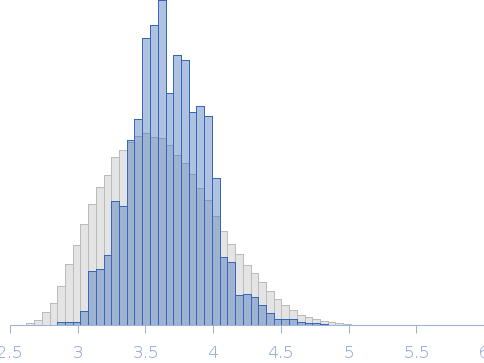 Rg, nm
Rg, nm