| MWexperimental | 10 | kDa |
| MWexpected | 14 | kDa |
| VPorod | 23 | nm3 |
|
log I(s)
3.18×10-1
3.18×10-2
3.18×10-3
3.18×10-4
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
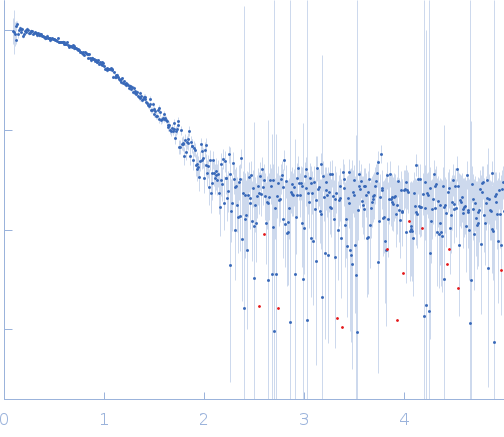
SAXS
data from solutions of
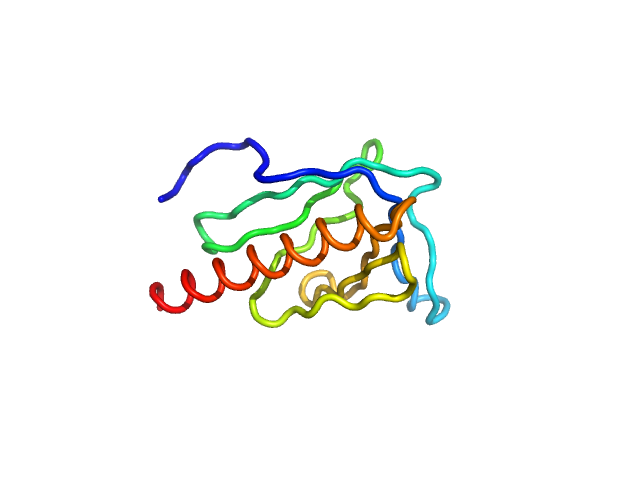
Enabled/vasodilator-stimulated phosphoprotein homology 1 domain (EVH1) from Mus musculus Homer1
in
20mM NaCl, 50 mM NaPi, 0.02% of sodium-azide, pH 7.4
were collected
on the
Rigaku BioSAXS-2000 instrument (CEITEC, Brno, Czech Republic)
using a Rigaku HyPix-3000 detector
at a sample-detector distance of 0.5 m and
at a wavelength of λ = 0.154 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
One solute concentration of 2.00 mg/ml was measured
at 25°C.
Six successive
600 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
|||||||||||||||||||||||||||