The two non-visual arrestins engage ERK2 differently.
Perry-Hauser NA,
Bennett Hopkins J,
Zhuo Y,
Zheng C,
Perez I,
Schultz KM,
Vishnivetskiy SA,
Kaya AI,
Sharma P,
Dalby KN,
Young Chung K,
Klug CS,
Gurevich VV,
Iverson TM
J Mol Biol
:167465
(2022 Jan 22)
|
|
|
|
|
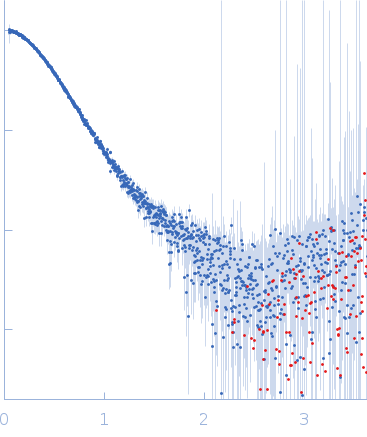
| Sample: |
Arrestin-3 fused to extracellular signal-regulated kinase 2 monomer, 89 kDa Bos taurus/Rattus norvegicus protein
|
| Buffer: |
20 mM MOPS pH 7.5, 150 mM NaCl, 1 mM TCEP, and 5% glycerol, pH: 7.5 |
| Experiment: |
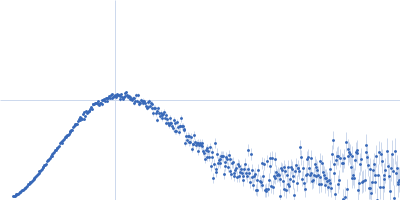
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2019 Mar 23
|
|
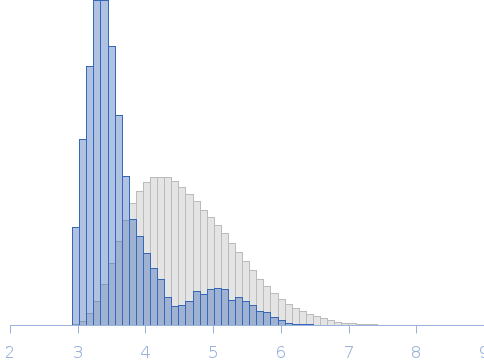
| RgGuinier |
3.6 |
nm |
| Dmax |
14.0 |
nm |
|
|
|
|
|
|
|
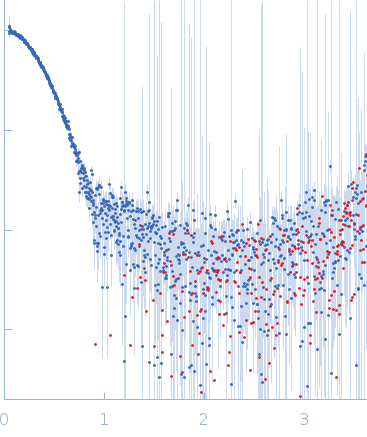
| Sample: |
Arrestin-3 fused to extracellular signal-regulated kinase 2 dimer, 178 kDa Bos taurus/Rattus norvegicus protein
|
| Buffer: |
20 mM MOPS pH 7.5, 150 mM NaCl, 1 mM TCEP, and 5% glycerol, pH: 7.5 |
| Experiment: |
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2019 Mar 23
|
|
| RgGuinier |
4.3 |
nm |
| Dmax |
16.0 |
nm |
|
|