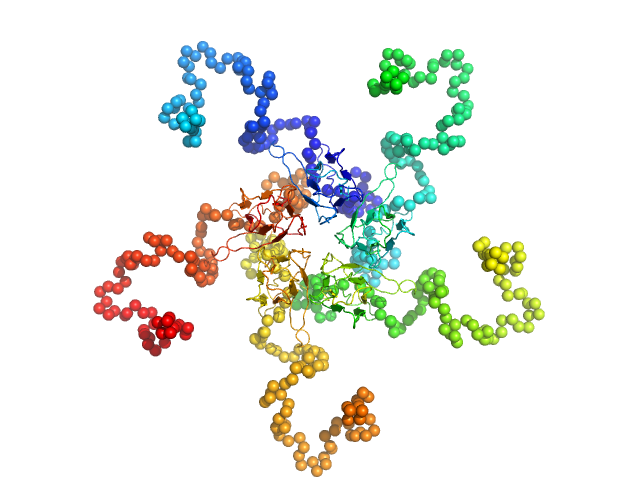A mechanism for histone chaperoning activity of nucleoplasmin: thermodynamic and structural models.
Taneva SG,
Bañuelos S,
Falces J,
Arregi I,
Muga A,
Konarev PV,
Svergun DI,
Velázquez-Campoy A,
Urbaneja MA
J Mol Biol
393(2):448-63
(2009 Oct 23)
|
|
|
|
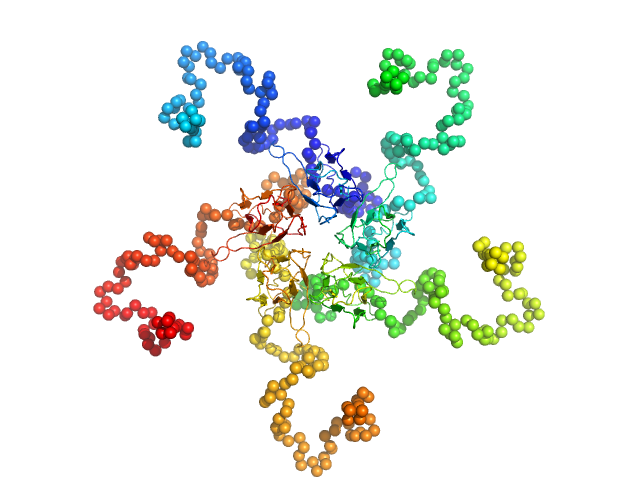
|
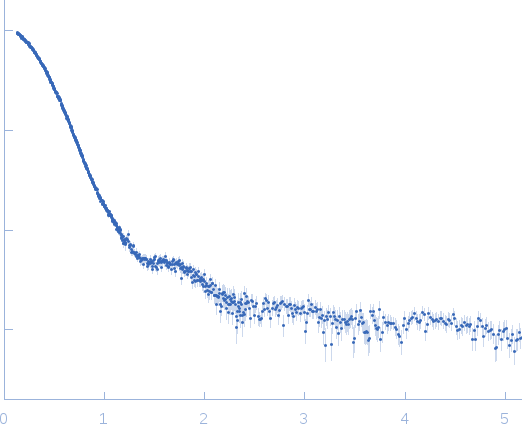
| Sample: |
Nucleoplasmin pentamer, 110 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Dec 3
|
|
| RgGuinier |
4.0 |
nm |
| Dmax |
12.6 |
nm |
| VolumePorod |
210 |
nm3 |
|
|
|
|
|
|
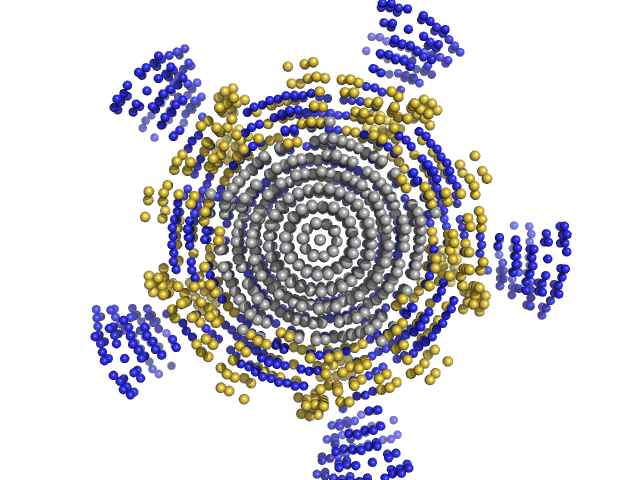
|
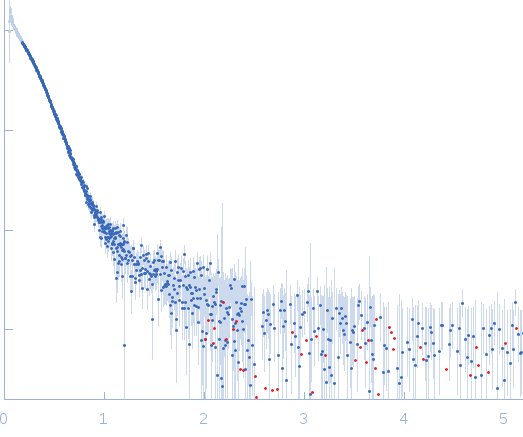
| Sample: |
NP-H5 pentamer, 200 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Dec 3
|
|
| RgGuinier |
5.2 |
nm |
| Dmax |
16.7 |
nm |
| VolumePorod |
410 |
nm3 |
|
|
|
|
|
|
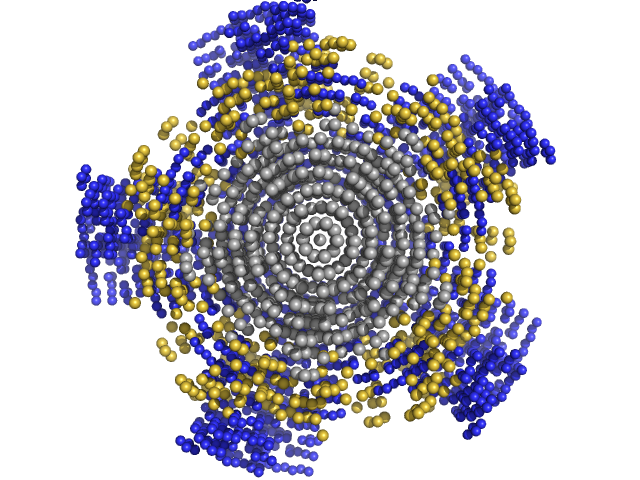
|
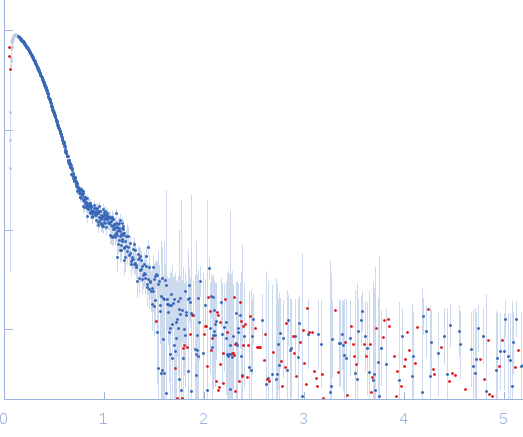
| Sample: |
NP-H2AH2B pentamer, 250 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2014 Dec 3
|
|
| RgGuinier |
4.7 |
nm |
| Dmax |
14.5 |
nm |
| VolumePorod |
470 |
nm3 |
|
|