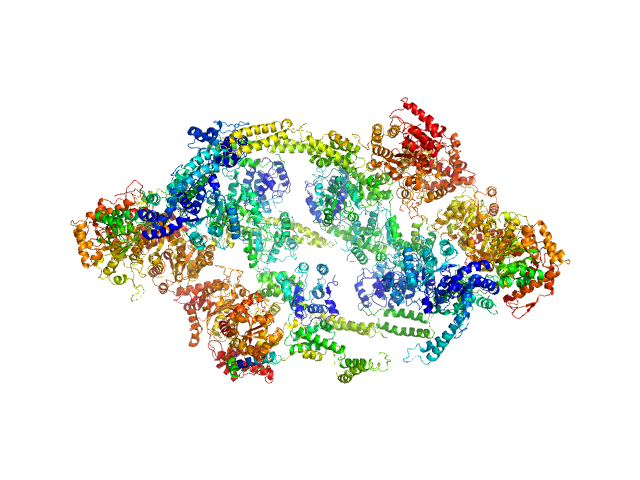
The antibiotic cyclomarin blocks arginine-phosphate-induced millisecond dynamics in the N-terminal domain of ClpC1 from Mycobacterium tuberculosis.
Weinhäupl K,
Brennich M,
Kazmaier U,
Lelievre J,
Ballell L,
Goldberg A,
Schanda P,
Fraga H
J Biol Chem
293(22):8379-8393
(2018 Jun 1)
|
|
|
|
|
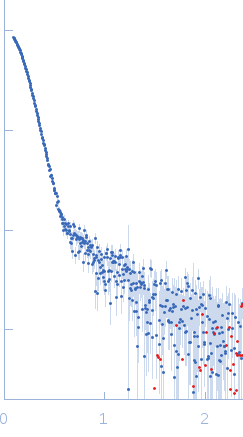
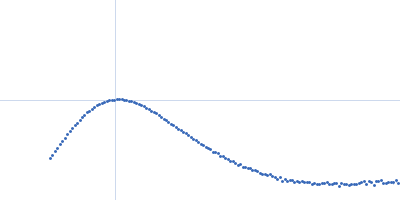
| Sample: |
ATP-dependent Clp protease ATP-binding subunit ClpC1, 95 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
Hepes 50 mM pH 7.5, KCl 100 mM, glycerol 10%, MgCl2 4 mM and ATP 1 mM, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Sep 18
|
|
| RgGuinier |
7.6 |
nm |
| Dmax |
25.0 |
nm |
| VolumePorod |
2156 |
nm3 |
|
|
|
|
|
|
|
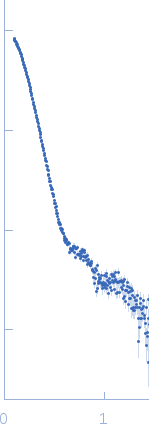
| Sample: |
ATP-dependent Clp protease ATP-binding subunit ClpC1, 95 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
Hepes 50 mM pH 7.5, KCl 100 mM, glycerol 10%, MgCl2 4 mM and ATP 1 mM, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Sep 18
|
|
| RgGuinier |
7.9 |
nm |
| Dmax |
25.1 |
nm |
| VolumePorod |
2416 |
nm3 |
|
|