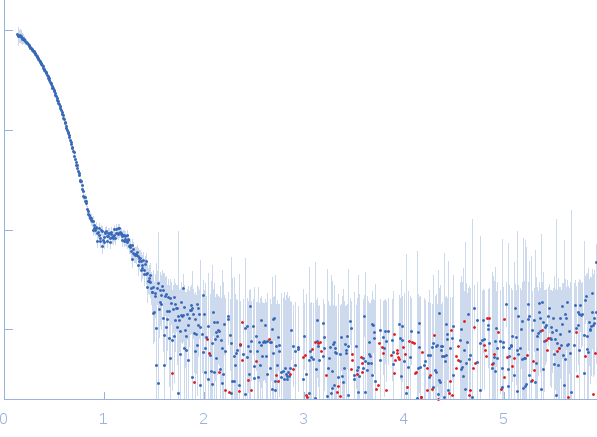
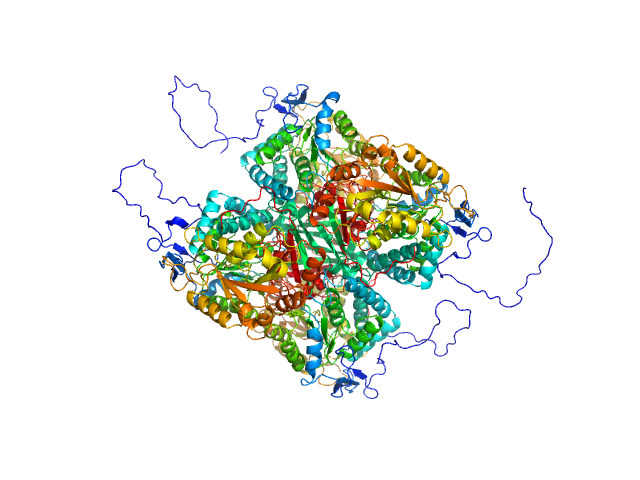
Structural and Biochemical Characterization of Aldehyde Dehydrogenase 12, the Last Enzyme of Proline Catabolism in Plants.
Korasick DA, Končitíková R, Kopečná M, Hájková E, Vigouroux A, Moréra S, Becker DF, Šebela M, Tanner JJ, Kopečný DJ Mol Biol (2018 Dec 20)
|
PMID: 30580036
doi: 10.1016/j.jmb.2018.12.010 |
Submitted to SASBDB: 2018 Oct 9 Published in SASBDB: |
|
|||||||||||||||||||||||