|
|
|
|
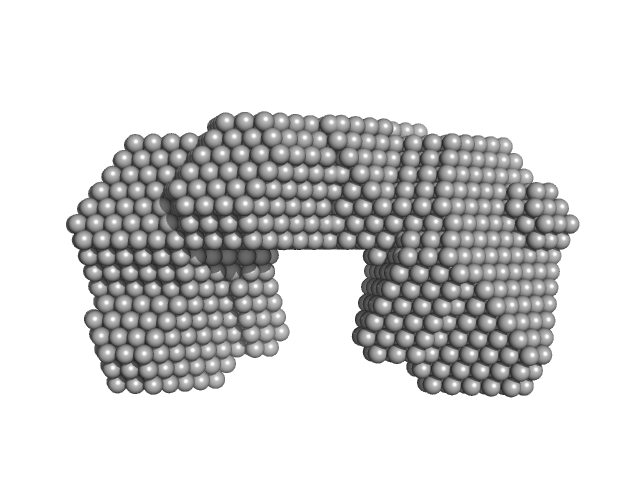
|
| Sample: |
Bifunctional protein PutA dimer, 219 kDa Bdellovibrio bacteriovorus protein
|
| Buffer: |
50 mM Tris, 125 mM NaCl, 1 mM EDTA, and 1 mM tris(3-hydroxypropyl)phosphine (THP) at pH 7.5,, pH: 7.5
|
| Experiment: |
SAXS
data collected at 12.3.1 (SIBYLS), Advanced Light Source (ALS) on 2012 Jun 8
|
Biophysical investigation of type A PutAs reveals a conserved core oligomeric structure.
FEBS J 284(18):3029-3049 (2017)
Korasick DA, Singh H, Pemberton TA, Luo M, Dhatwalia R, Tanner JJ
|
| RgGuinier |
4.5 |
nm |
| Dmax |
14.0 |
nm |
| VolumePorod |
287 |
nm3 |
|