Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase.
Thomsen M,
Tuukkanen A,
Dickerhoff J,
Palm GJ,
Kratzat H,
Svergun DI,
Weisz K,
Bornscheuer UT,
Hinrichs W
Acta Crystallogr D Biol Crystallogr
71(Pt 4):907-17
(2015 Apr)
|
|
|
|
|
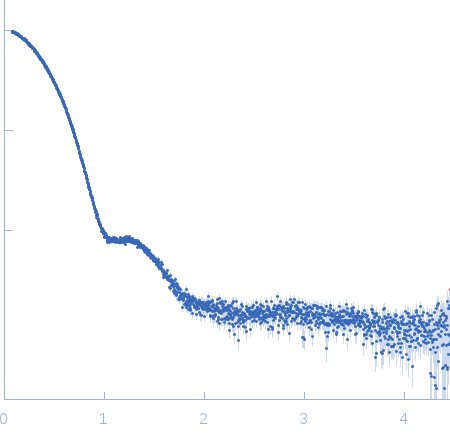
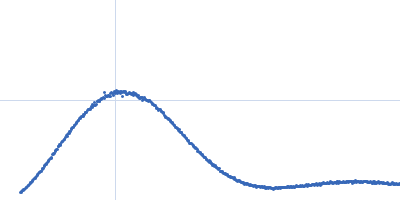
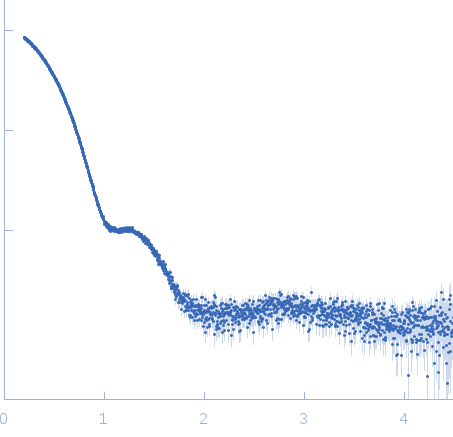
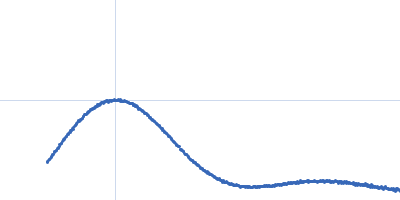
| Sample: |
Bacterial chalcone isomerase hexamer, 194 kDa Eubacterium ramulus protein
|
| Buffer: |
50 mM sodium phosphate, pH: 6.8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
|
| RgGuinier |
4.0 |
nm |
| Dmax |
13.0 |
nm |
| VolumePorod |
320 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Chalcone isomerase with Naringenin hexamer, 194 kDa Eubacterium ramulus protein
|
| Buffer: |
50 mM sodium phosphate, pH: 6.8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
|
| RgGuinier |
3.7 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
320 |
nm3 |
|
|
|
|
|
|
|
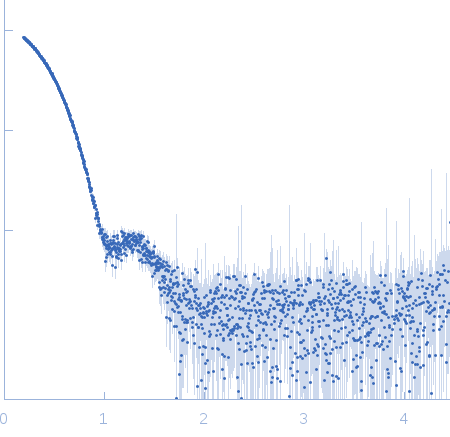
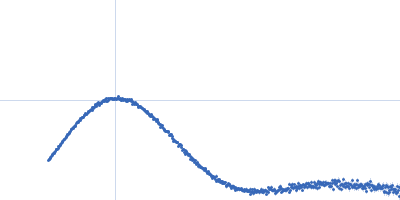
| Sample: |
Chalcone isomerase deltaLid hexamer, 181 kDa Eubacterium ramulus protein
|
| Buffer: |
50 mM sodium phosphate, pH: 6.8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
|
| RgGuinier |
3.6 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
270 |
nm3 |
|
|