|
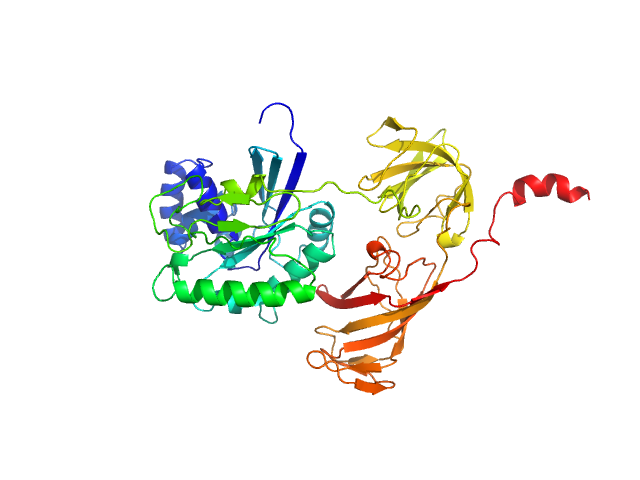
SAXS
data from solutions of
Mammalian translation elongation factor eEF1A2
in
25 mM Tris HCl, 150 mM NaCl, 6 mM βME, 20% glycerol, 0.01mM GDP,, pH 7.5
were collected
on the
Bruker Nanostar w Excillum source instrument (Department of Chemistry, iNANO building, Aarhus Uinversity, Aarhus C, Denmark)
using a VÅNTEC-2000 detector
at a sample-detector distance of 0.9 m and
at a wavelength of λ = 0.134 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
One solute concentration of 1.90 mg/ml was measured
at 20°C.
One
1800 second frame was collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
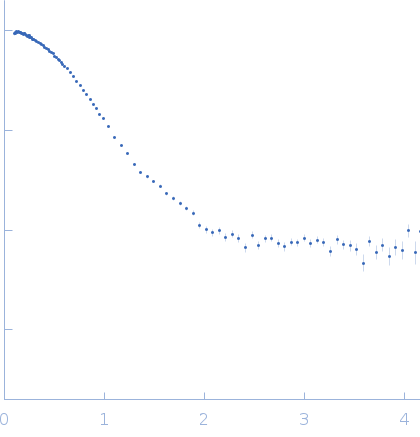 s, nm-1
s, nm-1