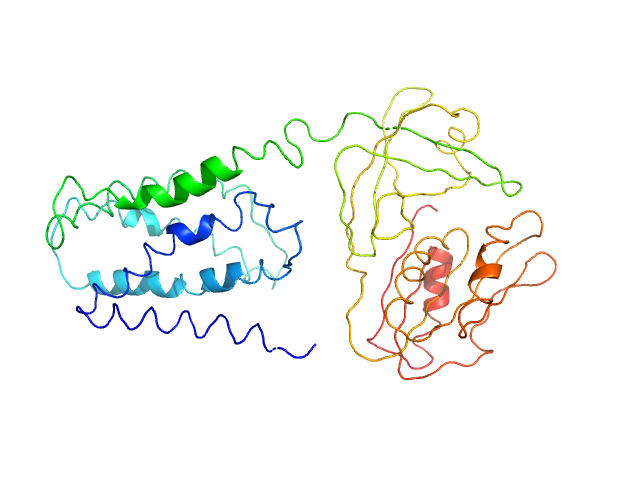
|
SANS data from solutions of Tag-free SpNOX in 50 mM Tris-HCl pH 7, 300 mM NaCl, 5 mM lauryl maltose neopentyl glycol (LMNG), 10 µM FAD, and 21.4% v/v D₂O were collected on the D22 instrument at the ILL (Grenoble, France) using a Reuter-Stokes 3He 128 linear sensitive multidetector. One solute concentration of approximately 1.60 mg/ml was measured at 7°C. There is a large uncertainty on the concentration due to the presence of several cofactors bound to the protein. The SANS signal of these samples was measured in Hellma suprasil quartz cells 100QS with 1 mm optical path length. The sample temperature was kept at 7 °C during the exposure times. Scattering data (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle) were recorded at two instrumental detector/collimator configurations, 2 m/2.8 m and 5.6 m/5.6 m, using a neutron wavelength of λ = 6 Å ± 10 %. At each instrumental configuration, the neutron flux reaching the sample was precisely measured, as well as the direct beam transmission, the scattering of a Boron-enriched material, the scattering and transmission of an empty cell, of all buffers and of all samples. The exposure times varied between 20 and 120 min. Data are scaled to absolute intensity using direct beam flux.
|
|
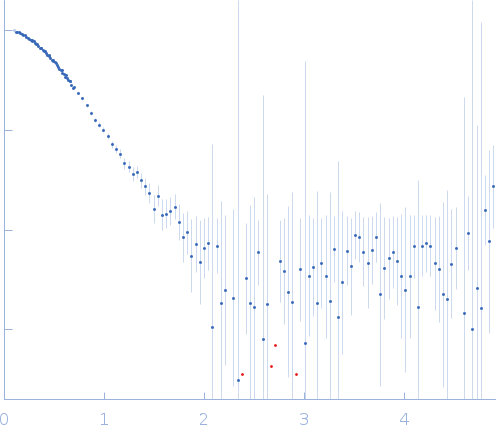 s, nm-1
s, nm-1