|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
High affinity immunoglobulin epsilon receptor subunit gamma monomer, 2 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.0 |
nm |
| VolumePorod |
54 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
T-cell surface glycoprotein CD3 gamma chain monomer, 3 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.1 |
nm |
| VolumePorod |
56 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
TYRO protein tyrosine kinase-binding protein monomer, 2 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.1 |
nm |
| VolumePorod |
53 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Endoplasmic reticulum chaperone BiP monomer, 42 kDa Homo sapiens protein
|
| Buffer: |
phosphate buffered saline, pH: 7.2 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2021 Mar 21
|
Structural basis of CDNF interaction with the UPR regulator GRP78.
Nat Commun 15(1):8175 (2024)
Graewert MA, Volkova M, Jonasson K, Määttä JAE, Gräwert T, Mamidi S, Kulesskaya N, Evenäs J, Johnsson RE, Svergun D, Bhattacharjee A, Huttunen HJ
|
| RgGuinier |
2.2 |
nm |
| Dmax |
6.5 |
nm |
| VolumePorod |
70 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Endoplasmic reticulum chaperone BiP monomer, 42 kDa Homo sapiens protein
Cerebral dopamine neurotrophic factor monomer, 21 kDa Homo sapiens protein
|
| Buffer: |
phosphate buffered saline, pH: 7.2 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2021 Mar 21
|
Structural basis of CDNF interaction with the UPR regulator GRP78.
Nat Commun 15(1):8175 (2024)
Graewert MA, Volkova M, Jonasson K, Määttä JAE, Gräwert T, Mamidi S, Kulesskaya N, Evenäs J, Johnsson RE, Svergun D, Bhattacharjee A, Huttunen HJ
|
| RgGuinier |
2.8 |
nm |
| Dmax |
10.0 |
nm |
| VolumePorod |
75 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Endoplasmic reticulum chaperone BiP monomer, 42 kDa Homo sapiens protein
C-terminal of the Cerebral dopamine neurotrophic factor monomer, 7 kDa Homo sapiens protein
|
| Buffer: |
phosphate buffered saline, pH: 7.2 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2021 Mar 23
|
Structural basis of CDNF interaction with the UPR regulator GRP78.
Nat Commun 15(1):8175 (2024)
Graewert MA, Volkova M, Jonasson K, Määttä JAE, Gräwert T, Mamidi S, Kulesskaya N, Evenäs J, Johnsson RE, Svergun D, Bhattacharjee A, Huttunen HJ
|
| RgGuinier |
2.4 |
nm |
| Dmax |
6.9 |
nm |
| VolumePorod |
66 |
nm3 |
|
|
|
|
|
|
|
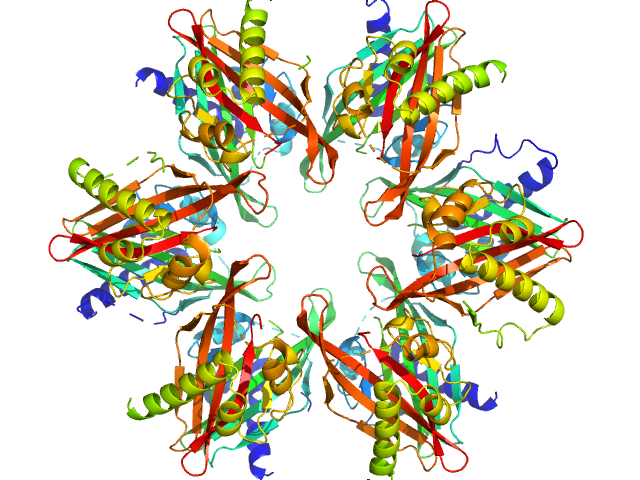
| Sample: |
Calcium/calmodulin-dependent protein kinase type II subunit alpha dodecamer, 186 kDa Homo sapiens protein
|
| Buffer: |
20 mM MES, 150 mM NaCl, 1 mM DTT, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2022 Oct 3
|
Ligand‐induced CaMKIIα
hub Trp403 flip, hub domain stacking, and modulation of kinase activity
Protein Science 33(10) (2024)
Narayanan D, Larsen A, Gauger S, Adafia R, Hammershøi R, Hamborg L, Bruus‐Jensen J, Griem‐Krey N, Gee C, Frølund B, Stratton M, Kuriyan J, Kastrup J, Langkilde A, Wellendorph P, Solbak S
|
| RgGuinier |
4.5 |
nm |
| Dmax |
15.2 |
nm |
| VolumePorod |
367 |
nm3 |
|
|
|
|
|
|
|
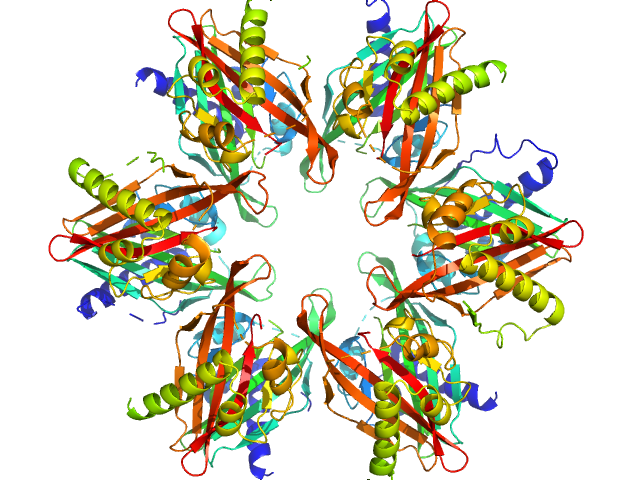
| Sample: |
Calcium/calmodulin-dependent protein kinase type II subunit alpha dodecamer, 186 kDa Homo sapiens protein
|
| Buffer: |
20 mM MES, 150 mM NaCl, 1 mM DTT, pH: 6 |
| Experiment: |
SAXS
data collected at Xenocs BioXolver L with MetalJet, University of Copenhagen, Department of Drug Design and Pharmacology on 2020 Nov 19
|
Ligand‐induced CaMKIIα
hub Trp403 flip, hub domain stacking, and modulation of kinase activity
Protein Science 33(10) (2024)
Narayanan D, Larsen A, Gauger S, Adafia R, Hammershøi R, Hamborg L, Bruus‐Jensen J, Griem‐Krey N, Gee C, Frølund B, Stratton M, Kuriyan J, Kastrup J, Langkilde A, Wellendorph P, Solbak S
|
| RgGuinier |
4.4 |
nm |
| Dmax |
14.9 |
nm |
| VolumePorod |
351 |
nm3 |
|
|
|
|
|
|
|
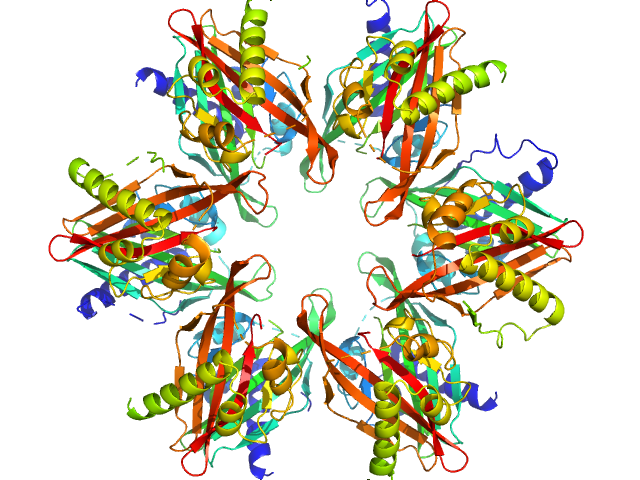
| Sample: |
Calcium/calmodulin-dependent protein kinase type II subunit alpha dodecamer, 186 kDa Homo sapiens protein
2-(6-(4-chlorophenyl)imidazo[1,2-b]pyridazine-2-yl)acetic acid monomer, 0 kDa
|
| Buffer: |
20 mM MES, 150 mM NaCl, 1 mM DTT, 200 µM PIPA, pH: 6 |
| Experiment: |
SAXS
data collected at Xenocs BioXolver L with MetalJet, University of Copenhagen, Department of Drug Design and Pharmacology on 2022 Nov 30
|
Ligand‐induced CaMKIIα
hub Trp403 flip, hub domain stacking, and modulation of kinase activity
Protein Science 33(10) (2024)
Narayanan D, Larsen A, Gauger S, Adafia R, Hammershøi R, Hamborg L, Bruus‐Jensen J, Griem‐Krey N, Gee C, Frølund B, Stratton M, Kuriyan J, Kastrup J, Langkilde A, Wellendorph P, Solbak S
|
| RgGuinier |
4.8 |
nm |
| Dmax |
16.0 |
nm |
| VolumePorod |
396 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Calcium/calmodulin-dependent protein kinase type II subunit alpha dodecamer, 186 kDa Homo sapiens protein
2-(6-(4-chlorophenyl)imidazo[1,2-b]pyridazine-2-yl)acetic acid monomer, 0 kDa
|
| Buffer: |
20 mM MES, 150 mM NaCl, 1 mM DTT, 200 µM PIPA, pH: 6 |
| Experiment: |
SAXS
data collected at Xenocs BioXolver L with MetalJet, University of Copenhagen, Department of Drug Design and Pharmacology on 2023 May 30
|
Ligand‐induced CaMKIIα
hub Trp403 flip, hub domain stacking, and modulation of kinase activity
Protein Science 33(10) (2024)
Narayanan D, Larsen A, Gauger S, Adafia R, Hammershøi R, Hamborg L, Bruus‐Jensen J, Griem‐Krey N, Gee C, Frølund B, Stratton M, Kuriyan J, Kastrup J, Langkilde A, Wellendorph P, Solbak S
|
| RgGuinier |
5.5 |
nm |
| Dmax |
27.0 |
nm |
|
|