|
|
|
|
|
| Sample: |
Glutamate/aspartate import solute-binding protein monomer, 32 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl, 20 mM NaPO4, 0.5 mM TCEP, pH: 7.4
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Sep 10
|
Structure-based screening of binding affinities via small-angle X-ray scattering
(2019)
Chen P, Masiewicz P, Perez K, Hennig J
|
| RgGuinier |
2.1 |
nm |
| Dmax |
6.4 |
nm |
| VolumePorod |
39 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
farnesylated human Guanylate-binding protein 1 monomer, 69 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Apr 30
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
3.9 |
nm |
| Dmax |
13.0 |
nm |
| VolumePorod |
102 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
human Guanylate-binding protein 1 monomer, 69 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Apr 30
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
3.9 |
nm |
| Dmax |
13.4 |
nm |
| VolumePorod |
106 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
human Guanylate-binding protein 1 dimer, 138 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, 0.2 mM GppNHp, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Apr 30
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
5.5 |
nm |
| Dmax |
22.0 |
nm |
| VolumePorod |
270 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
human Guanylate-binding protein 1 monomer, 69 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, 0.2 mM GppNHp, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Apr 30
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
4.5 |
nm |
| Dmax |
20.5 |
nm |
| VolumePorod |
120 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
human Guanylate-binding protein 1 dimer, 138 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Apr 30
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
4.8 |
nm |
| Dmax |
15.6 |
nm |
| VolumePorod |
211 |
nm3 |
|
|
|
|
|
|
|
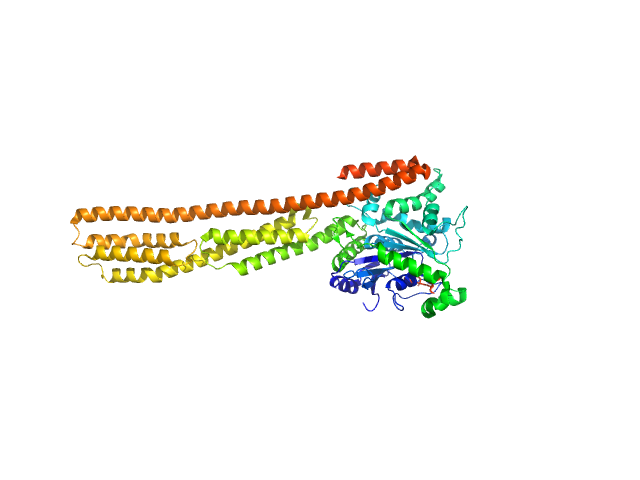
| Sample: |
farnesylated human Guanylate-binding protein 1 monomer, 69 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2016 May 8
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
3.8 |
nm |
| Dmax |
14.3 |
nm |
| VolumePorod |
102 |
nm3 |
|
|
|
|
|
|
|
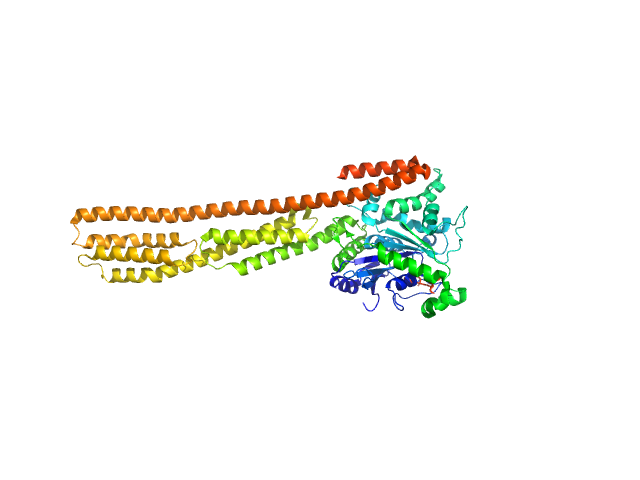
| Sample: |
human Guanylate-binding protein 1 monomer, 69 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2016 May 8
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
5.1 |
nm |
| Dmax |
27.4 |
nm |
| VolumePorod |
162 |
nm3 |
|
|
|
|
|
|
|
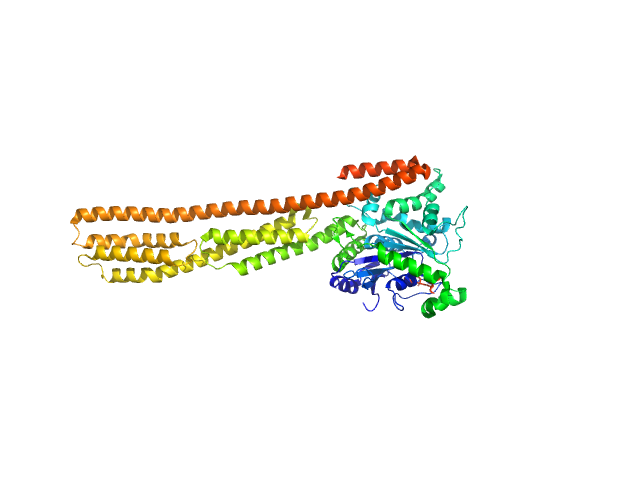
| Sample: |
human Guanylate-binding protein 1 monomer, 69 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 5 mM MgCl2, 150 mM NaCl, 0.2 mM GppNHp, pH: 7.9
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2016 May 8
|
Farnesylation of human guanylate binding protein 1 as safety mechanism preventing structural rearrangements and uninduced dimerization.
FEBS J (2019)
Lorenz C, Ince S, Zhang T, Cousin A, Batra-Safferling R, Nagel-Steger L, Herrmann C, Stadler AM
|
| RgGuinier |
5.5 |
nm |
| Dmax |
27.0 |
nm |
| VolumePorod |
186 |
nm3 |
|
|
|
|
|
|
|
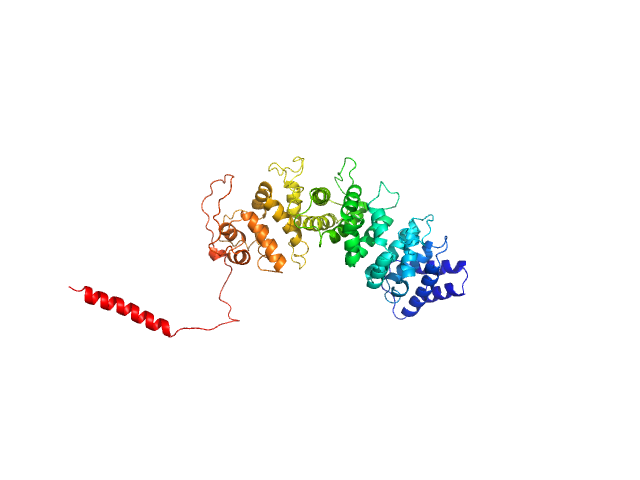
| Sample: |
Resistance to inhibitors of cholinesterase 8 homolog A monomer, 56 kDa Bos taurus protein
|
| Buffer: |
20 mM Tris, 150 mM KCl, 5 % glycerol, 1 mM TCEP, pH: 8
|
| Experiment: |
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2018 Oct 27
|
Structural underpinnings of Ric8A function as a G-protein α-subunit chaperone and guanine-nucleotide exchange factor.
Nat Commun 10(1):3084 (2019)
Srivastava D, Gakhar L, Artemyev NO
|
| RgGuinier |
3.4 |
nm |
| Dmax |
12.7 |
nm |
| VolumePorod |
88 |
nm3 |
|
|