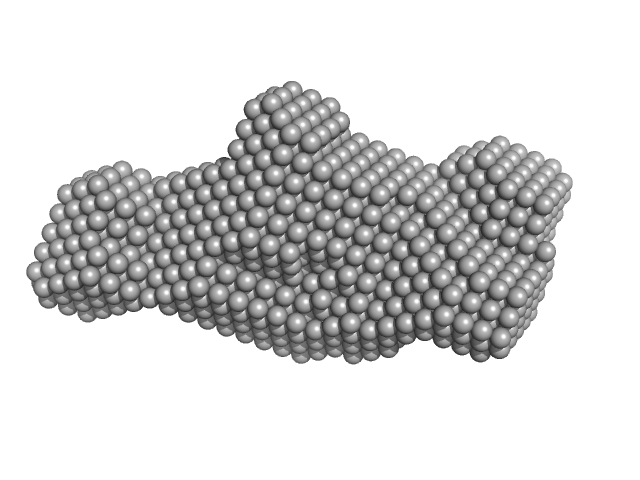
|
Synchrotron SAXS
data from solutions of
CHD4 (AH)
in
50 mM HEPES 50 mM KCl, pH 7.5
were collected
on the
EMBL X33 beam line
at the DORIS III, DESY storage ring
(Hamburg, Germany)
using a Pilatus 1M-W detector
at a sample-detector distance of 2.7 m and
at a wavelength of λ = 0.15 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
Solute concentrations ranging between 1.4 and 8.6 mg/ml were measured
at 10°C.
Eight successive
15 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The low angle data collected at lower concentrations were extrapolated to infinite dilution and merged with the higher concentration data to yield the final composite scattering curve.
The ATPase CHD4 mediates nucleosome remodeling by the NuRD (nucleosome remodeling and deacetylase) complex. The NuRD complex serves as a crucial epigenetic regulator of cell differentiation, proliferation, and hematopoietic development by coupling the deacetylation and demethylation of histones, nucleosome mobilization, and the recruitment of transcription factors. The three dimensional small-angle X-ray scattering model of CHD4 helps to define its interdomain interactions, with cross linking and limited proteolysis studies used to validate the model. Functional and binding assays suggest a regulatory role for the PHD and chromo domains.
|
|
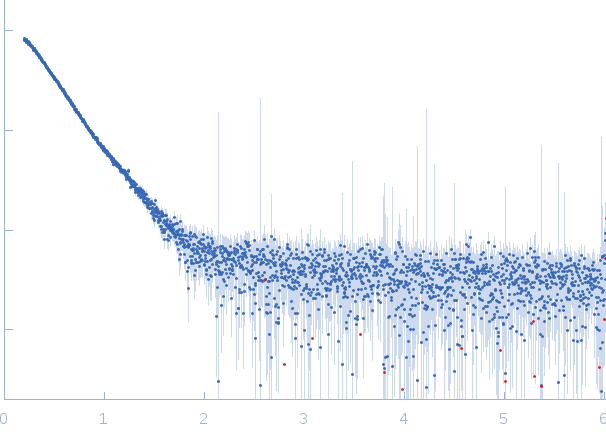 s, nm-1
s, nm-1