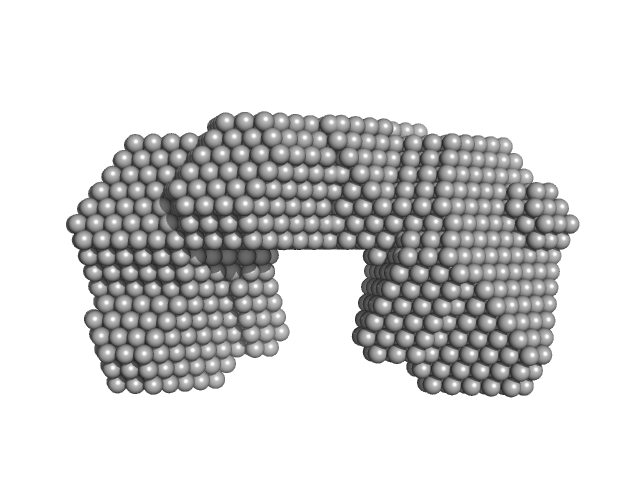
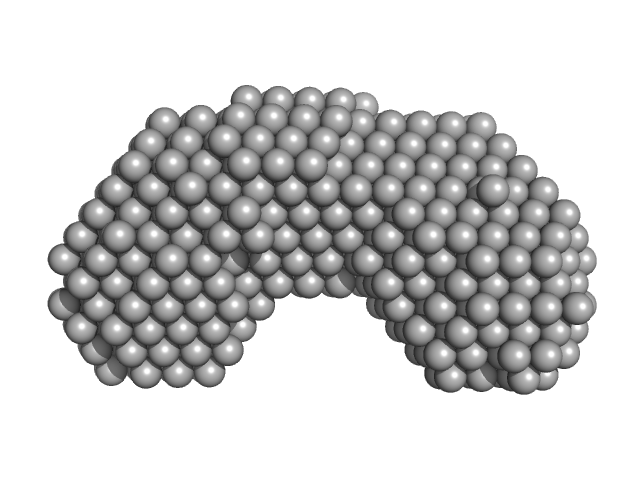
|
Synchrotron SAXS data from solutions of Proline utilization A from Bdellovibrio bacteriovorus in 50 mM Tris, 125 mM NaCl, 1 mM EDTA and 1 mM tris(3-hydroxypropyl)phosphine (THP) at pH 7.5 were collected on the 12.3.1 (SIBYLS) beam line at the Advanced Light Source (ALS; Berkeley, CA, USA) using a MAR 165 CCD detector. The data were normalized to the intensity of the transmitted beam and radially averaged. The radially averaged scattering of the solvent-blank was subtracted.
Wavelength = UNKNOWN. Cell temperature = UNKNOWN. Storage temperature = UNKNOWN. Sample detector distance = UNKNOWN. X-ray Exposure time = UNKNOWN. Number of frames = UNKNOWN. Concentration = UNKNOWN
|
|
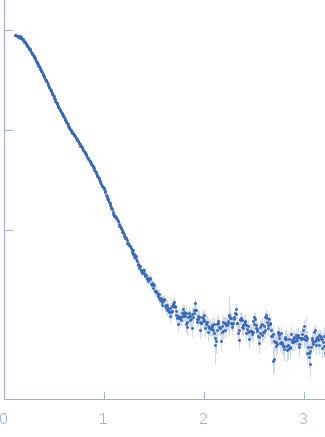 s, nm-1
s, nm-1