|
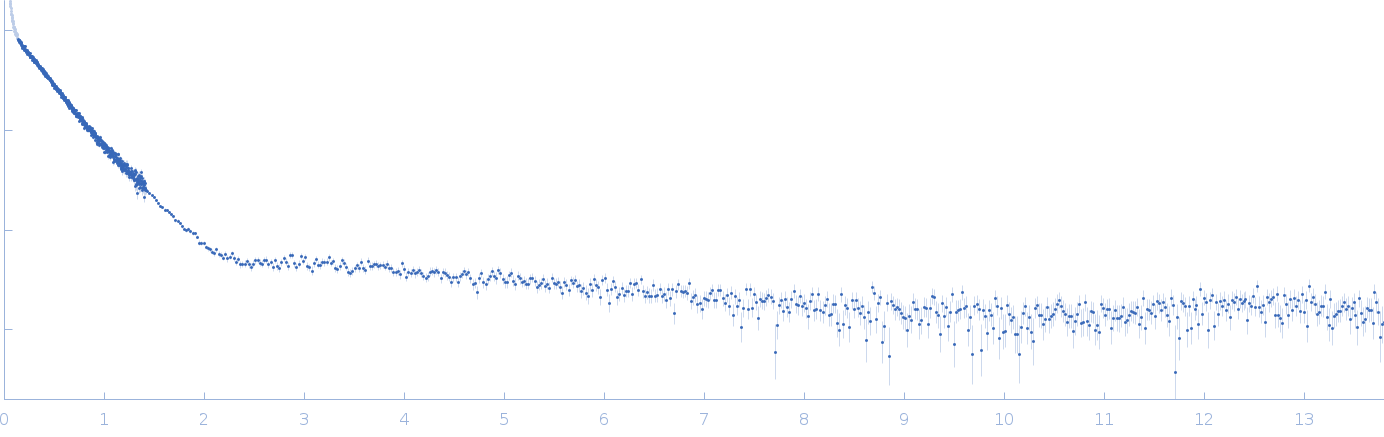
Synchrotron SAXS
data from solutions of
The Fe–S cluster assembly 1 homolog of pigeon (retention time at the magnet position No.5 : 60 min)
in
20 mM Tris-HCl, 0.15 M NaCl, 10 mM 3-mercapto-1,2-propanediol, pH 8
were collected
on the
BL-10C beam line
at the Photon Factory (PF), High Energy Accelerator Research Organization (KEK) storage ring
(Tsukuba, Japan)
using a Pilatus3 2M detector
at a sample-detector distance of 3 m and
at a wavelength of λ = 0.155 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
at 20°C.
One
60 second frame was collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
SAXS/WAXS data were measured at 20°C from a sample at 29.3 mg/ml using two different sample-to-detector distances (0.3 and 3 m), and merged using the program ATSAS 3.0.3. The sample solution was set at the position No. 4 in the periodic Nd-Fe-B permanent magnetic circuit [M. Hirai, M. Koizumi, R. Han, T. Hayakawa, Y. Sano, Right- /left-circular orientation of biological macromolecules under magnetic field gradient. J. Appl. Crystallogr. 36, 520–524 (2003)]. The retention time of a sample solution at the position No.5 in the magnet circuit was 60 min. P(r) was calculated from 0.016 to 0.518 Å-1 of I(q).
|
|
 s, nm-1
s, nm-1