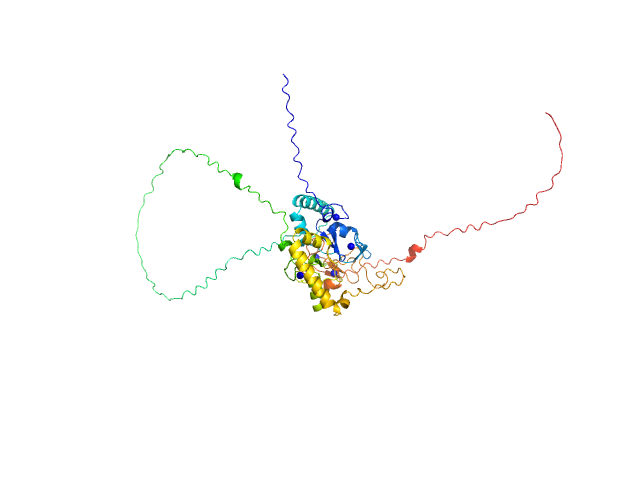
| MWI(0) | 44 | kDa |
| MWexpected | 46 | kDa |
| VPorod | 61 | nm3 |
|
log I(s)
1.64×10-1
1.64×10-2
1.64×10-3
1.64×10-4
|
 s, nm-1
s, nm-1
|
|
|
|
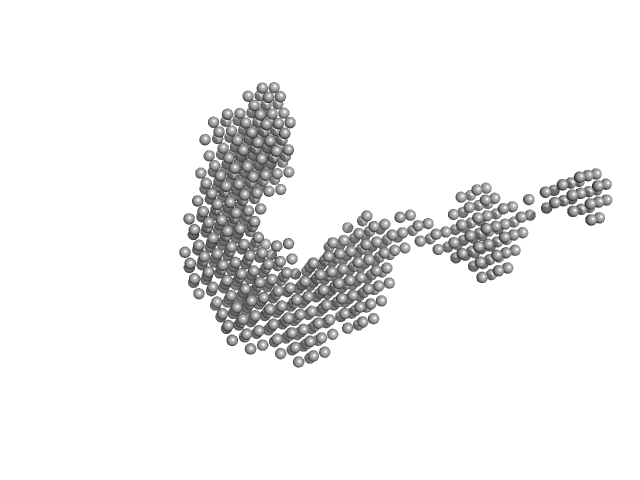
|
|
|
|
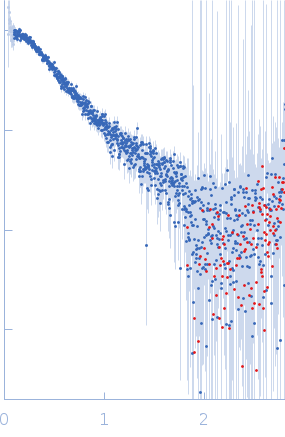
Synchrotron SAXS data from solutions of PRT1 in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM TCEP, and 2% (w/v) glycerol were collected on the BL-10C beam line at the Photon Factory (PF), High Energy Accelerator Research Organization (KEK; Tsukuba, Japan) using a Pilatus3 2M detector at a sample-detector distance of 3.0 m and at a wavelength of λ = 0.15 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 250.00 μl sample at 10 mg/ml was injected at different flow rates in the protein peak (20 sec/image with flow at 0.05 ml/min) and the base line (20 sec/image with flow at 0.5 ml/min) using a GE Superdex 200 Increase 10/300 column at 25°C. 332 successive 20-second frames were collected.
|
|
|||||||||||||||||||||||||||||||||