Structural determinants and mechanism of mammalian CRM1 allostery.
Dölker N,
Blanchet CE,
Voß B,
Haselbach D,
Kappel C,
Monecke T,
Svergun DI,
Stark H,
Ficner R,
Zachariae U,
Grubmüller H,
Dickmanns A
Structure
21(8):1350-60
(2013 Aug 6)
|
|
|
|
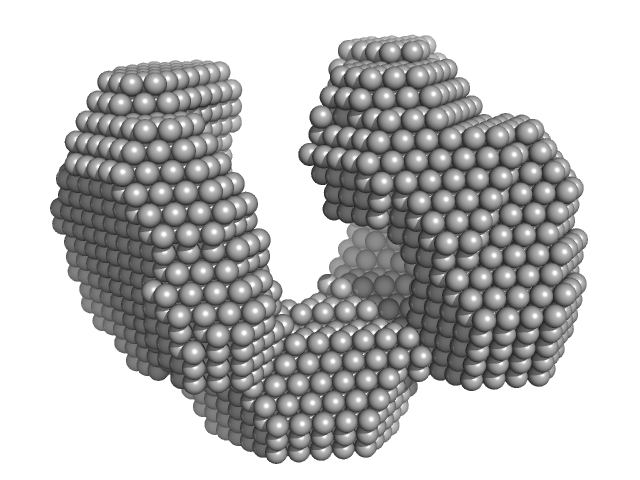
|
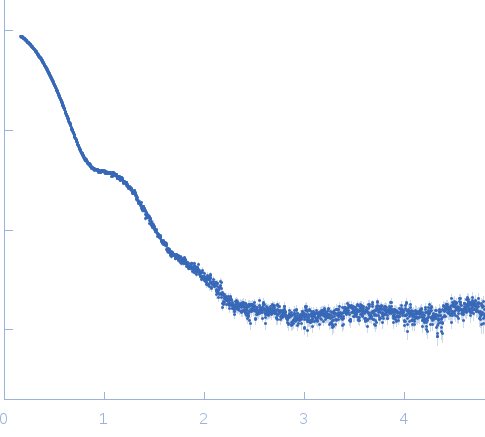
| Sample: |
Exportin-1 monomer, 123 kDa Mus musculus protein
|
| Buffer: |
50 mM Tris-HCL 150 mM NaCl 1.0 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2009 Feb 3
|
|
| RgGuinier |
3.8 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
190 |
nm3 |
|
|
|
|
|
|
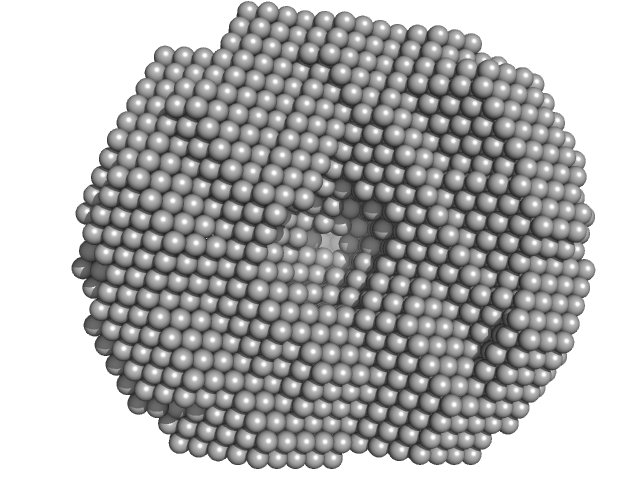
|
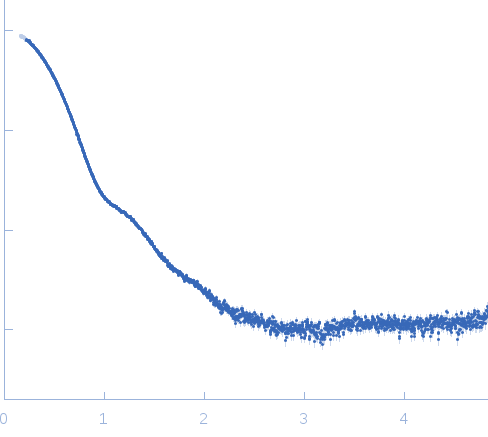
| Sample: |
Exportin-1 monomer, 123 kDa Mus musculus protein
GTP-binding nuclear protein Ran monomer, 24 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCL 150 mM NaCl 1.0 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2009 Feb 3
|
|
| RgGuinier |
3.6 |
nm |
| Dmax |
10.0 |
nm |
|
|
|
|
|
|
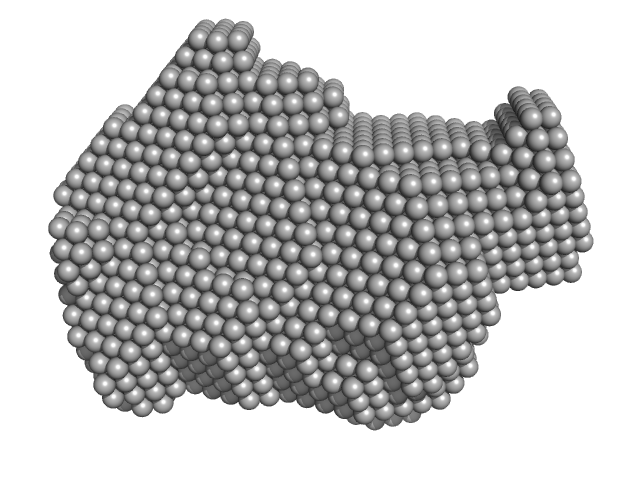
|
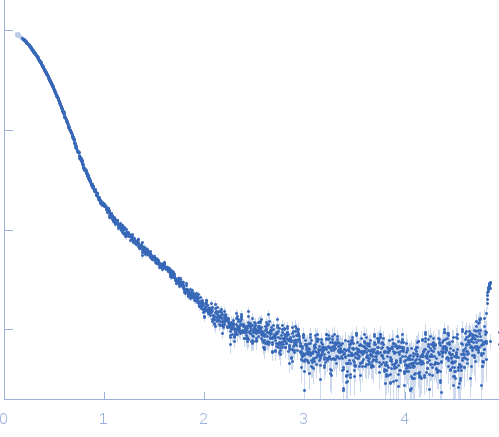
| Sample: |
Exportin-1 monomer, 123 kDa Mus musculus protein
GTP-binding nuclear protein Ran monomer, 24 kDa Homo sapiens protein
Snurportin-1 monomer, 41 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCL 150 mM NaCl 1.0 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2009 Feb 3
|
|
| RgGuinier |
4.1 |
nm |
| Dmax |
14.0 |
nm |
|
|
|
|
|
|
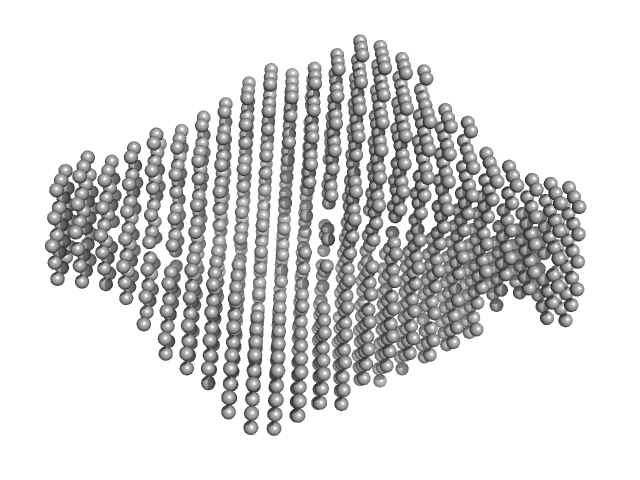
|
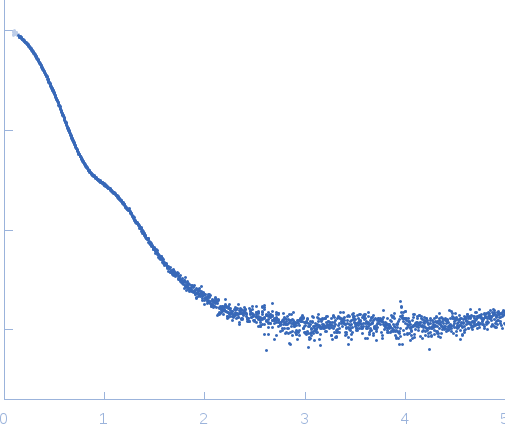
| Sample: |
Exportin-1 monomer, 123 kDa Mus musculus protein
Snurportin-1 monomer, 41 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCL 150 mM NaCl 1.0 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2011 Dec 10
|
|
| RgGuinier |
4.3 |
nm |
| Dmax |
15.0 |
nm |
|
|