Comparative studies of the low-resolution structure of two p23 co-chaperones for Hsp90 identified in Plasmodium falciparum genome.
Silva NSM,
Seraphim TV,
Minari K,
Barbosa LRS,
Borges JC
Int J Biol Macromol
108:193-204
(2018 Mar)
|
|
|
|
|
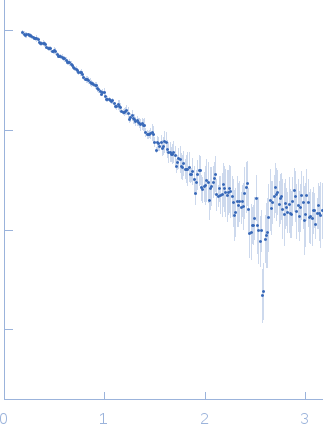
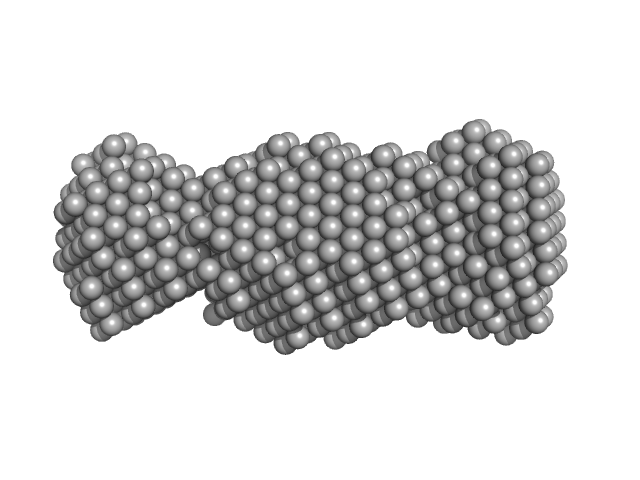
| Sample: |
CS domain protein, putative monomer, 19 kDa Plasmodium falciparum protein
|
| Buffer: |
25 mM Tris-HCl, 100 mM NaCl, 2 mM EDTA, 1 mM B-mercaptoethanol, pH: 7.4 |
| Experiment: |
SAXS
data collected at SAXS2 Beamline, Brazilian Synchrotron Light Laboratory on 2015 May 26
|
|
| RgGuinier |
2.5 |
nm |
| Dmax |
8.5 |
nm |
|
|
|
|
|
|
|
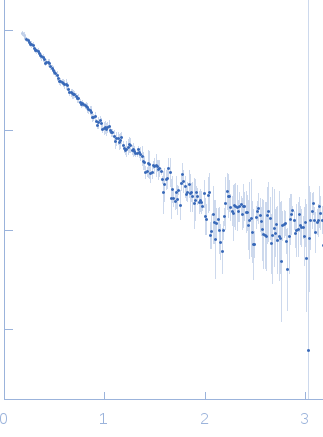
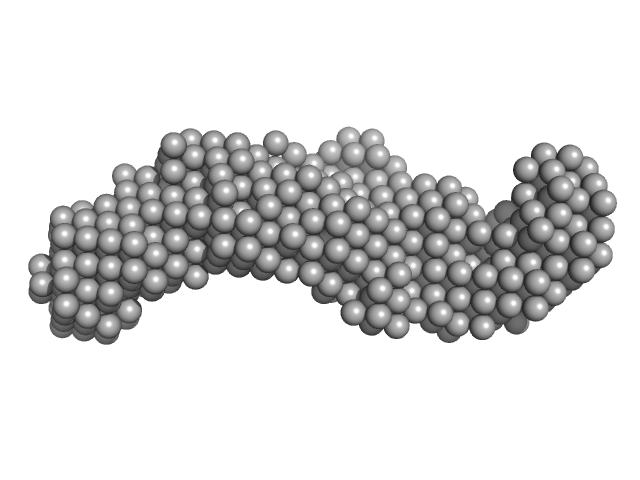
| Sample: |
Co-chaperone p23 monomer, 31 kDa Plasmodium falciparum protein
|
| Buffer: |
25 mM Tris-HCl, 100 mM NaCl, 2 mM EDTA, 1 mM B-mercaptoethanol, pH: 7.4 |
| Experiment: |
SAXS
data collected at SAXS2 Beamline, Brazilian Synchrotron Light Laboratory on 2015 May 26
|
|
| RgGuinier |
3.7 |
nm |
| Dmax |
13.0 |
nm |
|
|