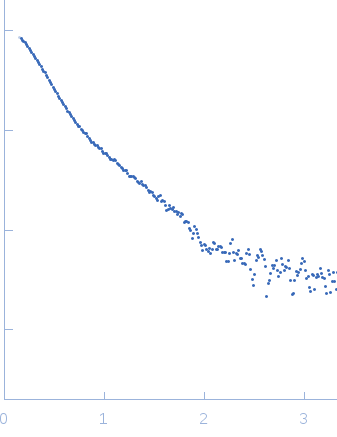
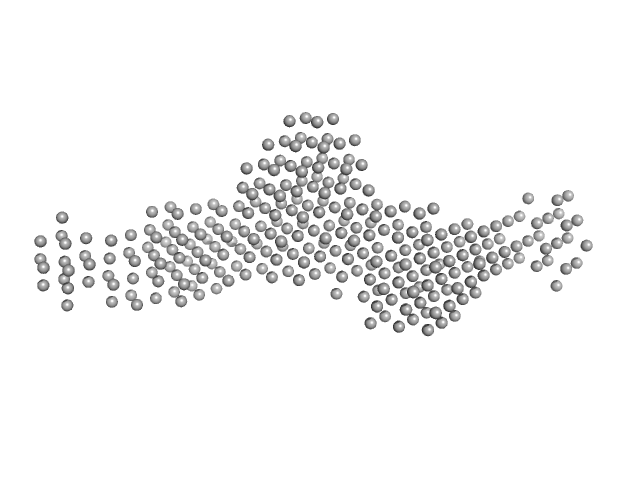
Structural and functional studies of the Leishmania braziliensis SGT co-chaperone indicate that it shares structural features with HIP and can interact with both Hsp90 and Hsp70 with similar affinities.
Coto ALS, Seraphim TV, Batista FAH, Dores-Silva PR, Barranco ABF, Teixeira FR, Gava LM, Borges JCInt J Biol Macromol 118(Pt A):693-706 (2018 Oct 15)
|
PMID: 29959008
doi: 10.1016/j.ijbiomac.2018.06.123 |
Submitted to SASBDB: 2017 Jun 17 Published in SASBDB: |
|
||||||||||||||||||||