|
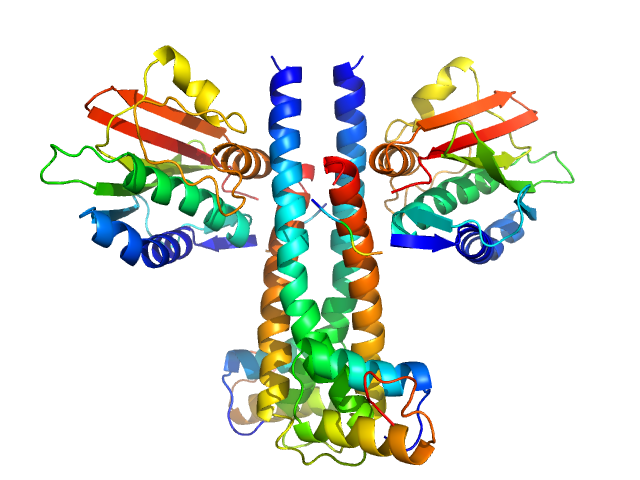
X-ray scattering data from the from the autokinase domain of the histidine kinase KinA complexed with the checkpoint inhibitor Sda in 50 mM Tris-HCl 200 mM NaCl 150 mM imidazole pH 8.5 were collected on a Bruker Nanostar instrument at the Bragg Institute (Australian Nuclear Science and Technology Organisation, Lucas Heights, Australia) using a HiStar 2D detector (I(s) vs s, where s = 4π sin θ/λ and 2θ is the scattering angle; λ=0.15406 nm). Approximately 15 µL of an 11.9 mg/ml protein solution was loaded into a quartz capillary mounted in a stainless steel holder. A single 3600s second frame was collected, and the buffer was collected in an analogous fashion. The data were radially averaged, and the scattering of the solvent-blank was subtracted. The data are on an arbitrary scale, and the mass of the protein was determined using a lysozyme secondary standard at a concentration of 17.5 mg/ml. Small-angle neutron scattering data with contrast variation were also collected on the NG3 instrument at the NIST Centre for Neutron Research at a solute concentration of ~11.9 mg/ml. A rigid-body model was co-refined against the SAXS and SANS data using using SASREF (v 7e), and this model is shown, together with the X-ray data and corresponding fit. Details of the SANS data collection, fits, and additional analysis are contained in a downloadable zip archive.
|
|
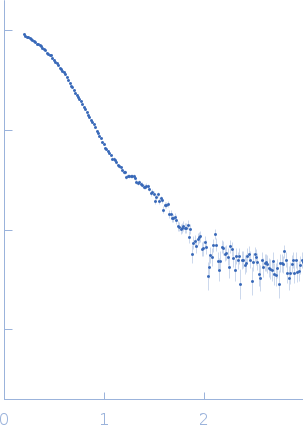 s, nm-1
s, nm-1