|
|
|
|

|
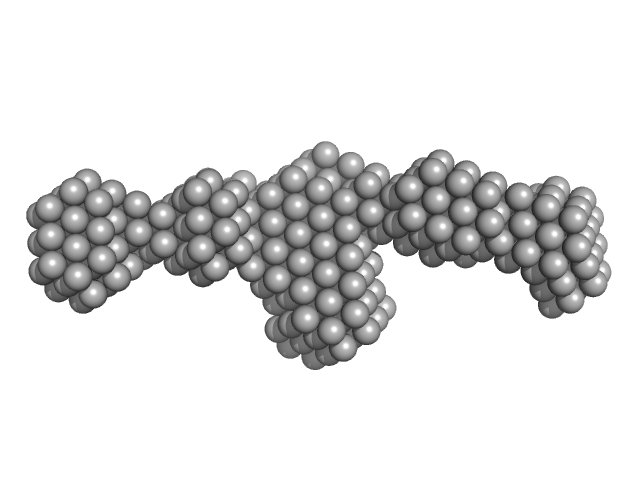
| Sample: |
Full-length SRP Alu RNA monomer, 38 kDa Plasmodium falciparum RNA
|
| Buffer: |
20 mM HEPES pH 7.5, 150 mM NaCl, 10 mM MgCl2, 10 mM KCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Jun 22
|
Structural analysis of the SRP Alu domain from Plasmodium falciparum reveals a non-canonical open conformation.
Commun Biol 4(1):600 (2021)
Soni K, Kempf G, Manalastas-Cantos K, Hendricks A, Flemming D, Guizetti J, Simon B, Frischknecht F, Svergun DI, Wild K, Sinning I
|
| RgGuinier |
3.3 |
nm |
| Dmax |
11.8 |
nm |
| VolumePorod |
63 |
nm3 |
|
|
|
|
|
|
|
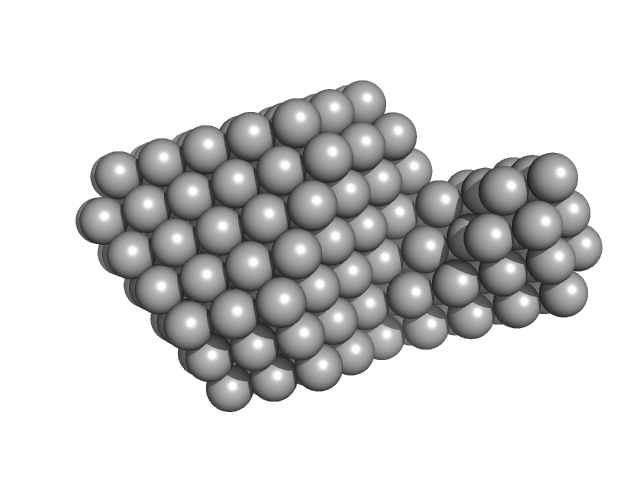
| Sample: |
SRP Alu RNA 5' domain monomer, 24 kDa Plasmodium falciparum RNA
|
| Buffer: |
20 mM HEPES pH 7.5, 150 mM NaCl, 10 mM MgCl2, 10 mM KCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Jun 22
|
Structural analysis of the SRP Alu domain from Plasmodium falciparum reveals a non-canonical open conformation.
Commun Biol 4(1):600 (2021)
Soni K, Kempf G, Manalastas-Cantos K, Hendricks A, Flemming D, Guizetti J, Simon B, Frischknecht F, Svergun DI, Wild K, Sinning I
|
| RgGuinier |
3.3 |
nm |
| Dmax |
11.5 |
nm |
| VolumePorod |
38 |
nm3 |
|
|
|
|
|
|
|
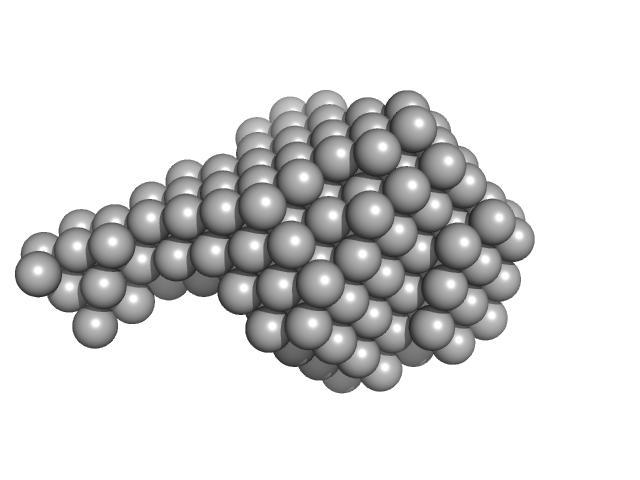
| Sample: |
Signal recognition particle 9 monomer, 12 kDa Plasmodium falciparum protein
Signal recognition particle 14 monomer, 12 kDa Plasmodium falciparum protein
Full-length SRP Alu RNA monomer, 38 kDa Plasmodium falciparum RNA
|
| Buffer: |
20 mM HEPES pH 7.5, 150 mM NaCl, 10 mM MgCl2, 10 mM KCl, 1mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Jun 22
|
Structural analysis of the SRP Alu domain from Plasmodium falciparum reveals a non-canonical open conformation.
Commun Biol 4(1):600 (2021)
Soni K, Kempf G, Manalastas-Cantos K, Hendricks A, Flemming D, Guizetti J, Simon B, Frischknecht F, Svergun DI, Wild K, Sinning I
|
| RgGuinier |
3.5 |
nm |
| Dmax |
12.0 |
nm |
| VolumePorod |
120 |
nm3 |
|
|
|
|
|
|
|
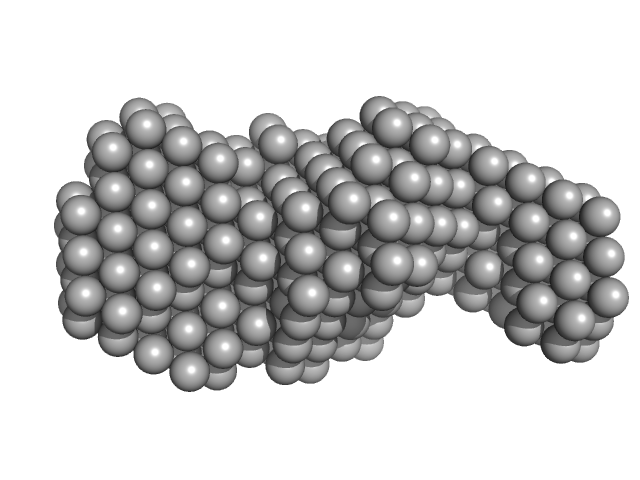
| Sample: |
Signal recognition particle 9 monomer, 12 kDa Plasmodium falciparum protein
Signal recognition particle 14 monomer, 12 kDa Plasmodium falciparum protein
SRP Alu RNA 5' domain monomer, 24 kDa Plasmodium falciparum RNA
|
| Buffer: |
20 mM HEPES pH 7.5, 150 mM NaCl, 10 mM MgCl2, 10 mM KCl, 1mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Jun 22
|
Structural analysis of the SRP Alu domain from Plasmodium falciparum reveals a non-canonical open conformation.
Commun Biol 4(1):600 (2021)
Soni K, Kempf G, Manalastas-Cantos K, Hendricks A, Flemming D, Guizetti J, Simon B, Frischknecht F, Svergun DI, Wild K, Sinning I
|
| RgGuinier |
3.2 |
nm |
| Dmax |
11.9 |
nm |
| VolumePorod |
77 |
nm3 |
|
|
|
|
|
|
|
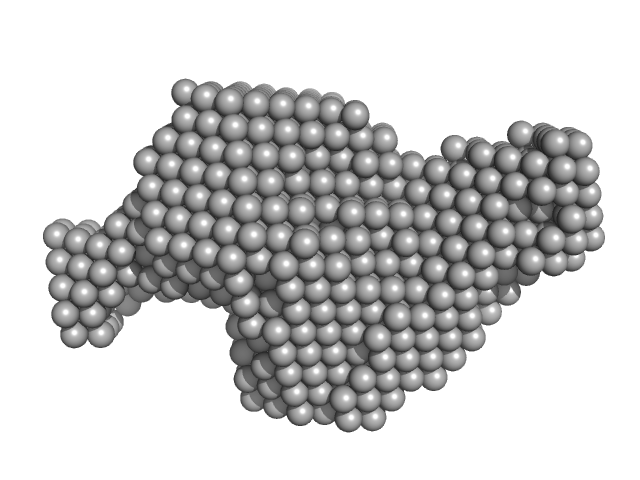
| Sample: |
Probable S-adenosyl-L-methionine-dependent RNA methyltransferase RSM22, mitochondrial monomer, 70 kDa Saccharomyces cerevisiae protein
|
| Buffer: |
40 mM Tris pH 7.5, 500 mM NaCl, 5% glycerol, 2.5 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2017 May 1
|
Expression and analysis of the SAM-dependent RNA methyltransferase Rsm22 from Saccharomyces cerevisiae
Acta Crystallographica Section D Structural Biology 77(6) (2021)
Alam J, Rahman F, Sah-Teli S, Venkatesan R, Koski M, Autio K, Hiltunen J, Kastaniotis A
|
| RgGuinier |
3.8 |
nm |
| Dmax |
13.9 |
nm |
| VolumePorod |
160 |
nm3 |
|
|
|
|
|
|
|
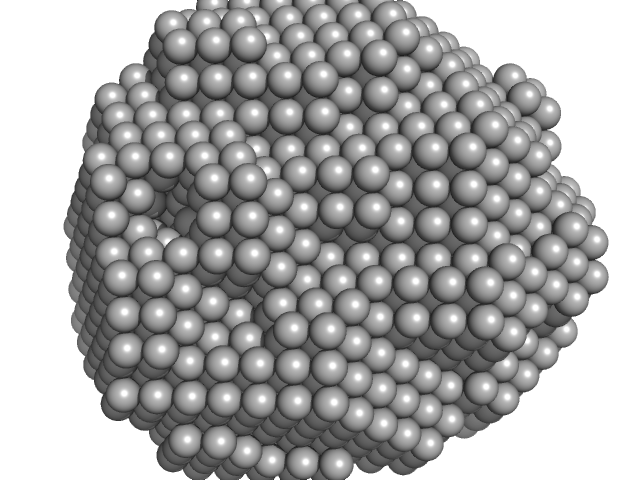
| Sample: |
Dimeric Probable S-adenosyl-L-methionine-dependent RNA methyltransferase RSM22, mitochondrial dimer, 141 kDa Saccharomyces cerevisiae protein
|
| Buffer: |
40 mM Tris pH 7.5, 500 mM NaCl, 5% glycerol, 2.5 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2017 May 1
|
Expression and analysis of the SAM-dependent RNA methyltransferase Rsm22 from Saccharomyces cerevisiae
Acta Crystallographica Section D Structural Biology 77(6) (2021)
Alam J, Rahman F, Sah-Teli S, Venkatesan R, Koski M, Autio K, Hiltunen J, Kastaniotis A
|
| RgGuinier |
5.0 |
nm |
| Dmax |
18.2 |
nm |
| VolumePorod |
516 |
nm3 |
|
|
|
|
|
|
|
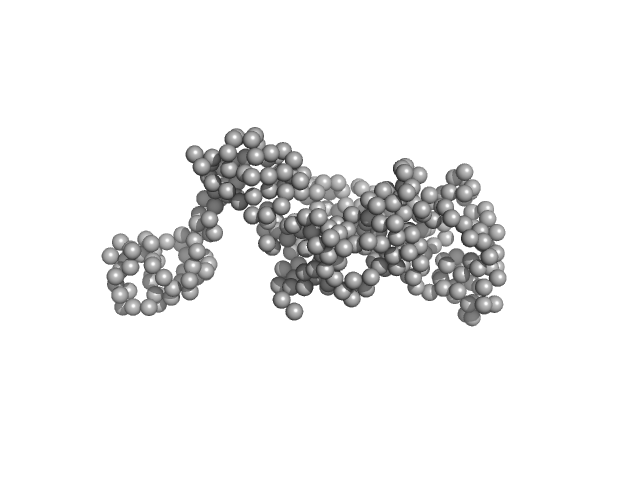
| Sample: |
DNA ligase A monomer, 38 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
50 mM Tris-HCl, 200 mM NaCl, 2 mM β-mercaptoethanol, pH: 8 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpace, CSIR-Central Drug Research Institute on 2018 Oct 6
|
Salt bridges at the subdomain interfaces of the adenylation domain and active-site residues of Mycobacterium tuberculosis
NAD +
-dependent DNA ligase A (MtbLigA) are important for the initial steps of nick-sealing activity
Acta Crystallographica Section D Structural Biology 77(6) (2021)
Afsar M, Shukla A, Kumar N, Ramachandran R
|
| RgGuinier |
2.4 |
nm |
| Dmax |
6.2 |
nm |
| VolumePorod |
94 |
nm3 |
|
|
|
|
|
|
|
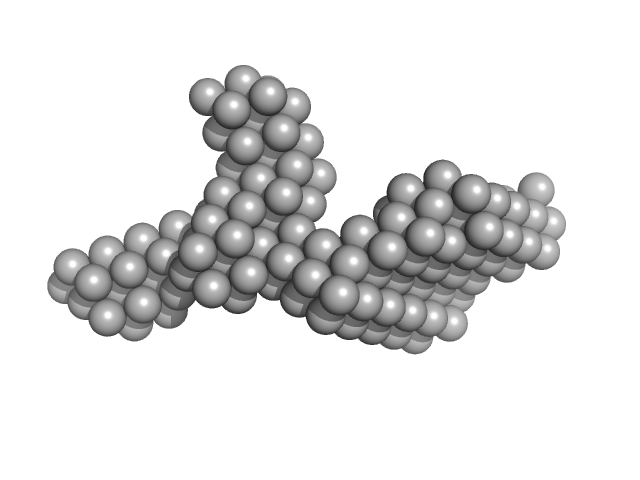
| Sample: |
DNA ligase A monomer, 37 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
50 mM Tris-HCl 200 mM NaCl 2mM β-mercaptoethanol, pH: 8 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpace, CSIR-Central Drug Research Institute on 2019 May 21
|
Salt bridges at the subdomain interfaces of the adenylation domain and active-site residues of Mycobacterium tuberculosis
NAD +
-dependent DNA ligase A (MtbLigA) are important for the initial steps of nick-sealing activity
Acta Crystallographica Section D Structural Biology 77(6) (2021)
Afsar M, Shukla A, Kumar N, Ramachandran R
|
| RgGuinier |
2.7 |
nm |
| Dmax |
8.9 |
nm |
| VolumePorod |
68 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Primer Binding Site-Segment monomer, 33 kDa HIV-1: pNL4-3 RNA
|
| Buffer: |
10 mM Tris, 140 mM KCl, 10 mM NaCl, 1 mM MgCl2, pH: 7.5 |
| Experiment: |
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2015 Jul 29
|
The three-way junction structure of the HIV-1 PBS-segment binds host enzyme important for viral infectivity.
Nucleic Acids Res (2021)
Song Z, Gremminger T, Singh G, Cheng Y, Li J, Qiu L, Ji J, Lange MJ, Zuo X, Chen SJ, Zou X, Boris-Lawrie K, Heng X
|
| RgGuinier |
3.4 |
nm |
| Dmax |
12.8 |
nm |
| VolumePorod |
87 |
nm3 |
|
|
|
|
|
|
|
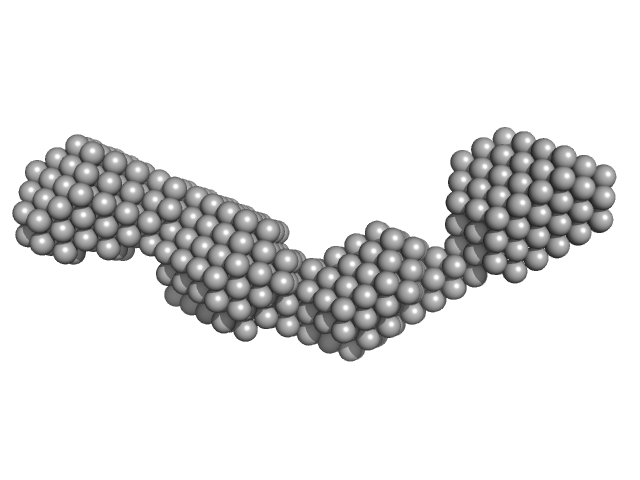
| Sample: |
Rationally optimised WA20 mutant N22A/H86K (ROWA) tetramer tetramer, 50 kDa Artificial protein (de … protein
|
| Buffer: |
20 mM HEPES, 150 mM NaCl, 5% glycerol,, pH: 7.5 |
| Experiment: |
SAXS
data collected at BL-10C, Photon Factory (PF), High Energy Accelerator Research Organization (KEK) on 2019 Nov 30
|
Rational thermostabilisation of four-helix bundle dimeric de novo proteins.
Sci Rep 11(1):7526 (2021)
Irumagawa S, Kobayashi K, Saito Y, Miyata T, Umetsu M, Kameda T, Arai R
|
| RgGuinier |
4.1 |
nm |
| Dmax |
18.1 |
nm |
| VolumePorod |
60 |
nm3 |
|
|