|
|
|
|
|
| Sample: |
Calcium/calmodulin-dependent protein kinase kinase 1 (M474L) monomer, 46 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 150 mM NaCl, 1 mM TCEP, 3% (w/v) glycerol, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2023 Aug 17
|
14-3-3 protein inhibits CaMKK1 by blocking the kinase active site with its last two C-terminal helices.
Protein Sci :e4805 (2023)
Petrvalska O, Honzejkova K, Koupilova N, Herman P, Obsilova V, Obsil T
|
| RgGuinier |
3.0 |
nm |
| Dmax |
10.5 |
nm |
| VolumePorod |
97 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Calcium/calmodulin-dependent protein kinase kinase 2 monomer, 48 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCl, 150 mM NaCl, 1 mM TCEP, 3% (w/v) glycerol, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2023 Aug 17
|
14-3-3 protein inhibits CaMKK1 by blocking the kinase active site with its last two C-terminal helices.
Protein Sci :e4805 (2023)
Petrvalska O, Honzejkova K, Koupilova N, Herman P, Obsilova V, Obsil T
|
| RgGuinier |
3.0 |
nm |
| Dmax |
12.0 |
nm |
| VolumePorod |
87 |
nm3 |
|
|
|
|
|
|
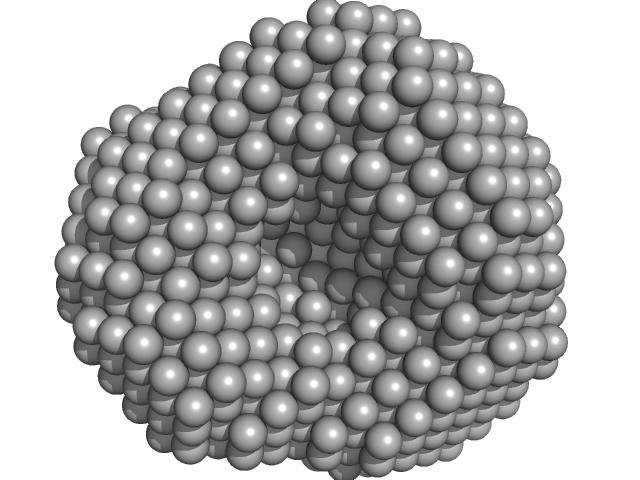
|
| Sample: |
Polyribonucleotide nucleotidyltransferase trimer, 246 kDa Campylobacter jejuni subsp. … protein
|
| Buffer: |
20 mM Tris-HCl, 10 mM NAH2PO4, 60 mM KCl, 1 mM MgCl2, 2 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2020 Mar 17
|
Structure and function of Campylobacter jejuni polynucleotide phosphorylase (PNPase): Insights into the role of this RNase in pathogenicity.
Biochimie (2023)
Bárria C, Athayde D, Hernandez G, Fonseca L, Casinhas J, Cordeiro TN, Archer M, Arraiano CM, Brito JA, Matos RG
|
| RgGuinier |
3.9 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
310 |
nm3 |
|
|
|
|
|
|
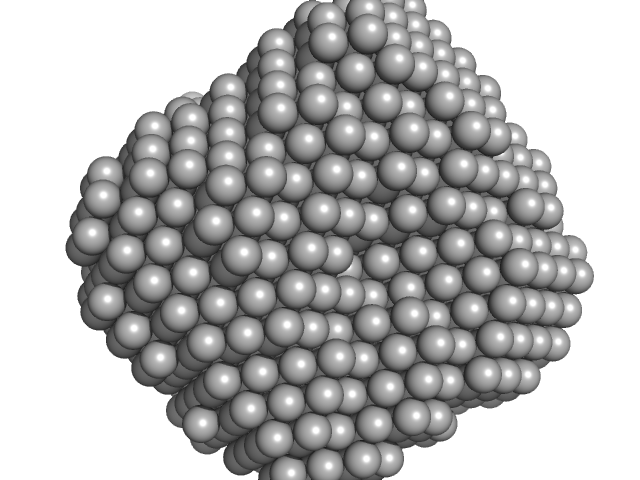
|
| Sample: |
Polyribonucleotide nucleotidyltransferase trimer, 237 kDa Campylobacter jejuni subsp. … protein
|
| Buffer: |
20 mM Tris.HCl, 10 mM NAH2PO4, 60 mM KCl, 1 mM MgCl2, 2 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2020 Mar 17
|
Structure and function of Campylobacter jejuni polynucleotide phosphorylase (PNPase): Insights into the role of this RNase in pathogenicity.
Biochimie (2023)
Bárria C, Athayde D, Hernandez G, Fonseca L, Casinhas J, Cordeiro TN, Archer M, Arraiano CM, Brito JA, Matos RG
|
| RgGuinier |
3.9 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
315 |
nm3 |
|
|
|
|
|
|
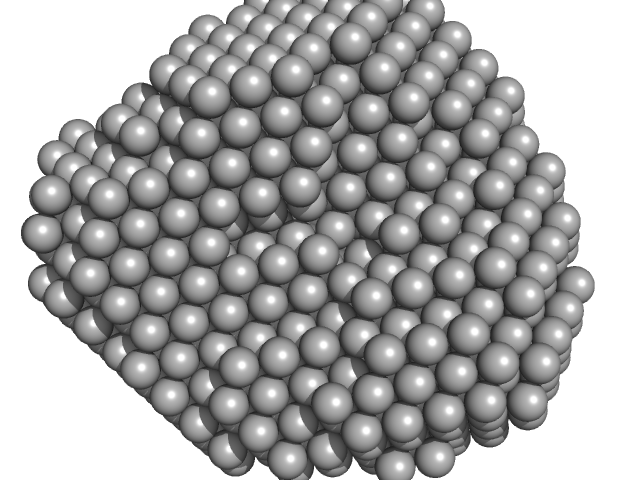
|
| Sample: |
Polyribonucleotide nucleotidyltransferase trimer, 237 kDa Campylobacter jejuni subsp. … protein
|
| Buffer: |
20 mM Tris.HCl, 10 mM NAH2PO4, 60 mM KCl, 1mM MgCl2, 2 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2020 Mar 17
|
Structure and function of Campylobacter jejuni polynucleotide phosphorylase (PNPase): Insights into the role of this RNase in pathogenicity.
Biochimie (2023)
Bárria C, Athayde D, Hernandez G, Fonseca L, Casinhas J, Cordeiro TN, Archer M, Arraiano CM, Brito JA, Matos RG
|
| RgGuinier |
3.9 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
312 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Beclin-1 (C18S, C21S, A103V, C137S, C140S) monomer, 17 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris, 300 mM NaCl, pH: 8 |
| Experiment: |
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 12
|
Invariant BECN1 CXXC motifs bind Zn(2+) and regulate structure and function of the BECN1 intrinsically disordered region.
Autophagy :1-17 (2023)
Mukhopadhyay S, Subedi S, Hopkins JB, Ugrinov A, Chakravarthy S, Colbert CL, Sinha SC
|
| RgGuinier |
4.1 |
nm |
| Dmax |
19.3 |
nm |
| VolumePorod |
57 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Beclin-1 monomer, 17 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris, 300 mM NaCl, pH: 8 |
| Experiment: |
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 12
|
Invariant BECN1 CXXC motifs bind Zn(2+) and regulate structure and function of the BECN1 intrinsically disordered region.
Autophagy :1-17 (2023)
Mukhopadhyay S, Subedi S, Hopkins JB, Ugrinov A, Chakravarthy S, Colbert CL, Sinha SC
|
| RgGuinier |
4.3 |
nm |
| Dmax |
20.0 |
nm |
| VolumePorod |
87 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Beclin-1 monomer, 17 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris, 300 mM NaCl, pH: 8 |
| Experiment: |
SAXS
data collected at BioCAT 18ID, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 12
|
Invariant BECN1 CXXC motifs bind Zn(2+) and regulate structure and function of the BECN1 intrinsically disordered region.
Autophagy :1-17 (2023)
Mukhopadhyay S, Subedi S, Hopkins JB, Ugrinov A, Chakravarthy S, Colbert CL, Sinha SC
|
| RgGuinier |
2.8 |
nm |
| Dmax |
12.0 |
nm |
| VolumePorod |
49 |
nm3 |
|
|
|
|
|
|
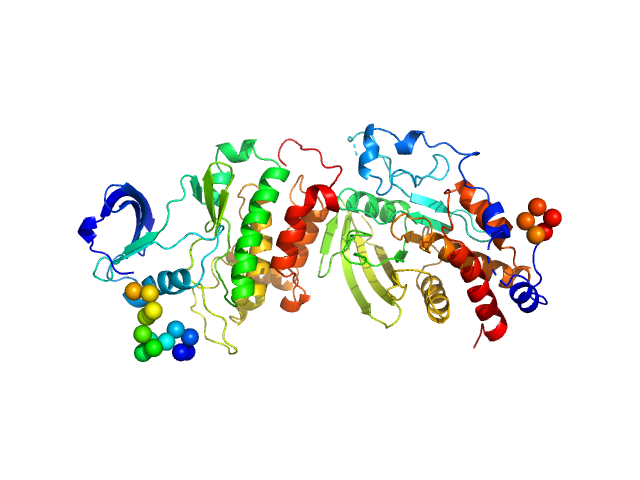
|
| Sample: |
Receptor-type tyrosine-protein phosphatase epsilon monomer, 34 kDa Homo sapiens protein
Proto-oncogene tyrosine-protein kinase Src, T357M mutant monomer, 31 kDa Homo sapiens protein
|
| Buffer: |
20 mM Tris , 50 mM NaCl, 5 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at 23A1, Taiwan Photon Source, NSRRC on 2017 May 26
|
An integrative approach unveils a distal encounter site for rPTPε and phospho-Src complex formation
Structure (2023)
EswarKumar N, Yang C, Tewary S, Peng W, Chen G, Yeh Y, Yang H, Ho M
|
| RgGuinier |
2.9 |
nm |
| Dmax |
10.0 |
nm |
| VolumePorod |
60 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Iron-sulfur cluster co-chaperone protein HscB monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris-HCl, 50 mM NaCl, 5 mM KCl, 2 mM β-mercaptoethanol, pH: 7.5 |
| Experiment: |
SAXS
data collected at SAXS1 Beamline, Brazilian Synchrotron Light Laboratory on 2018 Aug 14
|
Structural characterization of the human DjC20/HscB cochaperone in solution
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics :140970 (2023)
de Souza Coto A, Pereira A, Oliveira S, de Oliveira Moritz M, da Rocha A, Dores-Silva P, da Silva N, de Araújo Nogueira A, Gava L, Seraphim T, Borges J
|
| RgGuinier |
2.5 |
nm |
| Dmax |
9.0 |
nm |
| VolumePorod |
40 |
nm3 |
|
|