|
|
|
|
|
| Sample: |
Humanized immunoglobulin G4 monoclonal antibody (lebrikizumab) monomer, 148 kDa Mouse/human protein
|
| Buffer: |
100 mM glycine-HCl, 200 mM NaCl, pH: 2 |
| Experiment: |
SAXS
data collected at BL-10C, Photon Factory (PF), High Energy Accelerator Research Organization (KEK) on 2024 Nov 19
|
IgG4
and IgG1
undergo common acid‐induced compaction into an alternatively folded state
FEBS Letters (2025)
Imamura H, Honda S
|
| RgGuinier |
4.1 |
nm |
| Dmax |
14.0 |
nm |
| VolumePorod |
238 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Humanized immunoglobulin G1 monoclonal antibody (trastuzumab) monomer, 148 kDa Mouse/human protein
|
| Buffer: |
100 mM glycine-HCl, 200 mM NaCl, pH: 2 |
| Experiment: |
SAXS
data collected at BL-10C, Photon Factory (PF), High Energy Accelerator Research Organization (KEK) on 2023 May 20
|
IgG4
and IgG1
undergo common acid‐induced compaction into an alternatively folded state
FEBS Letters (2025)
Imamura H, Honda S
|
| RgGuinier |
4.2 |
nm |
| Dmax |
12.9 |
nm |
| VolumePorod |
240 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Humanized immunoglobulin G4 monoclonal antibody (lebrikizumab) monomer, 148 kDa Mouse/human protein
|
| Buffer: |
10 mM sodium phosphate, pH: 7 |
| Experiment: |
SAXS
data collected at BL-10C, Photon Factory (PF), High Energy Accelerator Research Organization (KEK) on 2024 Nov 19
|
IgG4
and IgG1
undergo common acid‐induced compaction into an alternatively folded state
FEBS Letters (2025)
Imamura H, Honda S
|
| RgGuinier |
4.8 |
nm |
| Dmax |
15.6 |
nm |
| VolumePorod |
211 |
nm3 |
|
|
|
|
|
|
|
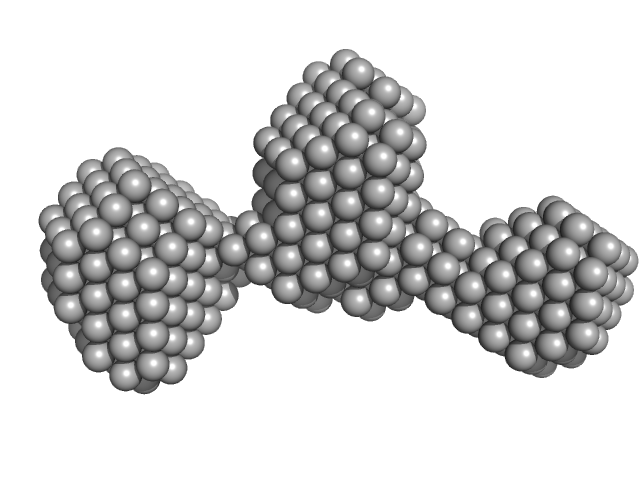
| Sample: |
LNA-DNA-LNA monomer, 4 kDa DNA
|
| Buffer: |
phosphate buffered saline, pH: 7.4 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2021 Oct 21
|
Application of enhanced biophysical strategies to determine ASO/target RNA binding affinity and kinetics
Michael Lerche
|
| RgGuinier |
1.3 |
nm |
| Dmax |
4.0 |
nm |
| VolumePorod |
4 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
MOE PS gapmers 3-8-3 monomer, 4 kDa DNA
|
| Buffer: |
phosphate buffered saline, pH: 7.4 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2021 Oct 21
|
Application of enhanced biophysical strategies to determine ASO/target RNA binding affinity and kinetics
Michael Lerche
|
| RgGuinier |
1.4 |
nm |
| Dmax |
4.3 |
nm |
| VolumePorod |
5 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
OME ASO monomer, 4 kDa RNA
|
| Buffer: |
phosphate buffered saline, pH: 7.4 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2021 Oct 21
|
Application of enhanced biophysical strategies to determine ASO/target RNA binding affinity and kinetics
Michael Lerche
|
| RgGuinier |
1.4 |
nm |
| Dmax |
4.0 |
nm |
| VolumePorod |
7 |
nm3 |
|
|
|
|
|
|
|
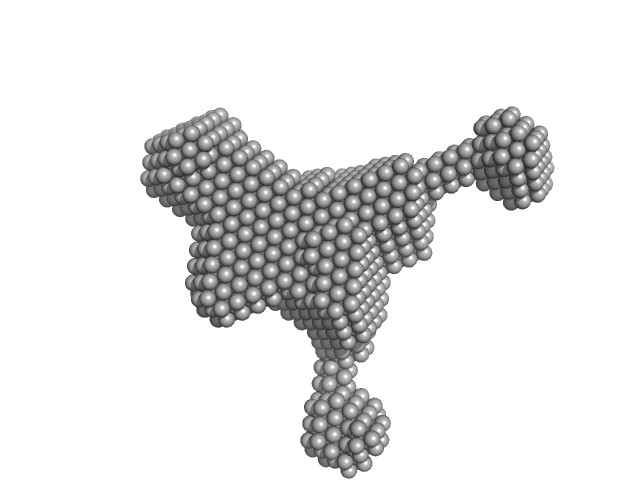
| Sample: |
LNA-DNA-LNA monomer, 4 kDa DNA
PSCK9, 24mer ASO binding site monomer, 8 kDa RNA
|
| Buffer: |
phosphate buffered saline, pH: 7.4 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2021 Oct 21
|
Application of enhanced biophysical strategies to determine ASO/target RNA binding affinity and kinetics
Michael Lerche
|
| RgGuinier |
1.9 |
nm |
| Dmax |
6.2 |
nm |
| VolumePorod |
15 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
MOE PS gapmers 3-8-3 monomer, 4 kDa DNA
PSCK9, 24mer ASO binding site monomer, 8 kDa RNA
|
| Buffer: |
phosphate buffered saline, pH: 7.4 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2021 Oct 21
|
Application of enhanced biophysical strategies to determine ASO/target RNA binding affinity and kinetics
Michael Lerche
|
| RgGuinier |
2.1 |
nm |
| Dmax |
6.4 |
nm |
| VolumePorod |
16 |
nm3 |
|
|
|
|
|
|
|
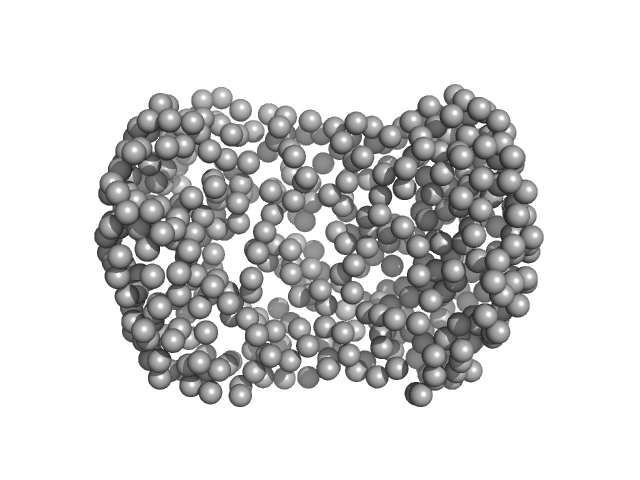
| Sample: |
OME ASO monomer, 4 kDa RNA
PSCK9, 24mer ASO binding site monomer, 8 kDa RNA
|
| Buffer: |
phosphate buffered saline, pH: 7.4 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2021 Oct 21
|
Application of enhanced biophysical strategies to determine ASO/target RNA binding affinity and kinetics
Michael Lerche
|
| RgGuinier |
1.9 |
nm |
| Dmax |
6.2 |
nm |
| VolumePorod |
16 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Auxin response factor monomer, 46 kDa Marchantia polymorpha protein
|
| Buffer: |
20 mM Tris-HCl, 150 mM NaCl, 1 mM DTT, pH: 8 |
| Experiment: |
SAXS
data collected at BL11 - NCD, ALBA on 2019 Dec 3
|
The structure and function of the DNA binding domain of class B MpARF2 share more traits with class A AtARF5 than to that of class B AtARF1.
Structure (2025)
Crespo I, Malfois M, Rienstra J, Tarrés-Solé A, van den Berg W, Weijers D, Boer DR
|
| RgGuinier |
2.6 |
nm |
| Dmax |
6.2 |
nm |
| VolumePorod |
59 |
nm3 |
|
|