|
In house laboratory SAXS data from a solution of the BRCT domain from M. tuberculosis DNA ligase in 50 mM Tris-HCl 500 mM NaCl 5mM β-mercaptoethanol, pH 8 were collected using an Anton Paar SAXSpace instrument at the CSIR-Central Drug Research Institute (Lucknow, India) using a Dectris Mythen 2R1K detector at a sample-detector distance of 0.3 m and at a wavelength of λ = 0.154 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 10.00 mg/ml was measured at 10°C. Two successive 1800 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
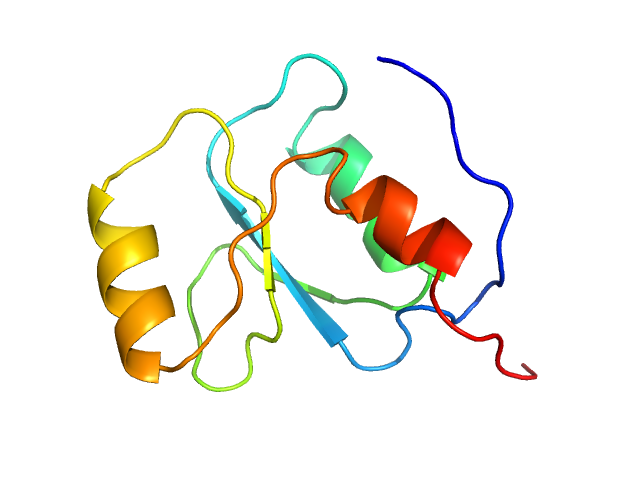
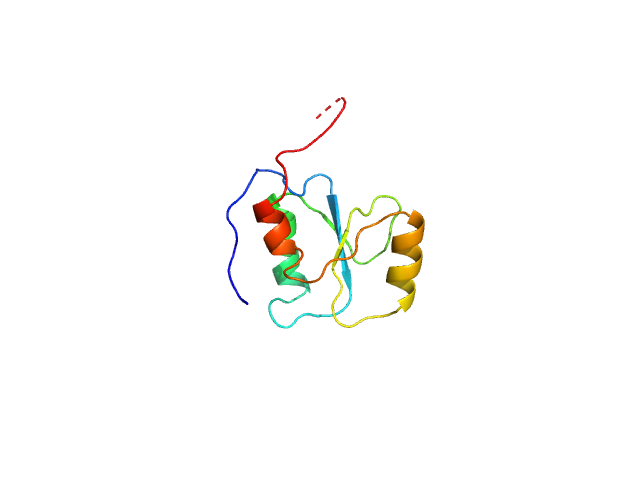
The experimental molecular weight quoted for this entry was evaluated using calibrated size-exclusion chromatography (GE Healthcare Superdex75 10/30 column). The atomistic models displayed in this entry (ribbon format) are a parent Phyre2 protein homology model (top) and one of ten structures obtained from the parent after elNémo normal mode calculations (bottom). Refer to: Kelley et al. (2015) Nature Protocols 10, 845-858; Suhre & Sanejouand (2004) Nucleic Acids Res. 32(Web Server issue): W610–W614.
|
|
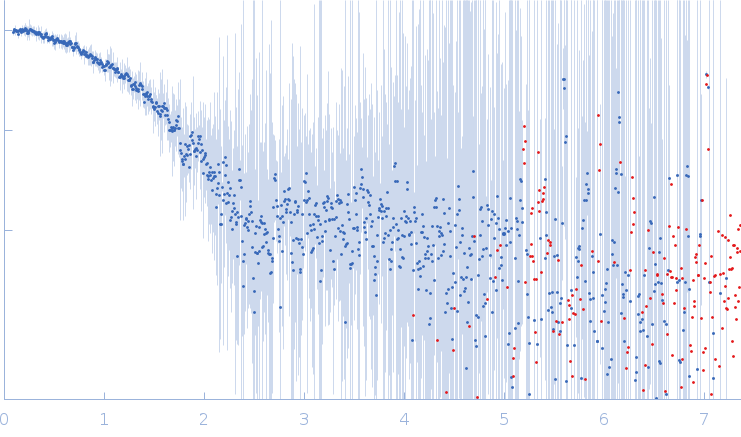 s, nm-1
s, nm-1