UniProt ID: P09651-2 (2-320) Isoform A1-A of Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Isoform A1-A of Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 34 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P09651 (1-196) Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P09651 (1-196) Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P09651 (1-196) Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P09651 (1-196) Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P09651 (1-196) Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P09651 (1-196) Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S )
|
|
|
|
| Sample: |
Heterogeneous nuclear ribonucleoprotein A1 (C43S/R75D/R88D/C175S ) monomer, 25 kDa Homo sapiens protein
|
| Buffer: |
100 mM HEPES, 350 mM NaCl, 0.5 mM EDTA, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2023 Mar 1
|
Thermodynamic coupling of the tandem RRM domains of hnRNP A1 underlie its pleiotropic RNA binding functions.
Sci Adv 10(28):eadk6580 (2024)
Levengood JD, Potoyan D, Penumutchu S, Kumar A, Zhou Q, Wang Y, Hansen AL, Kutluay S, Roche J, Tolbert BS
|
|
|
UniProt ID: P9WFC7 (22-719) UvrABC system protein B
|
|
|
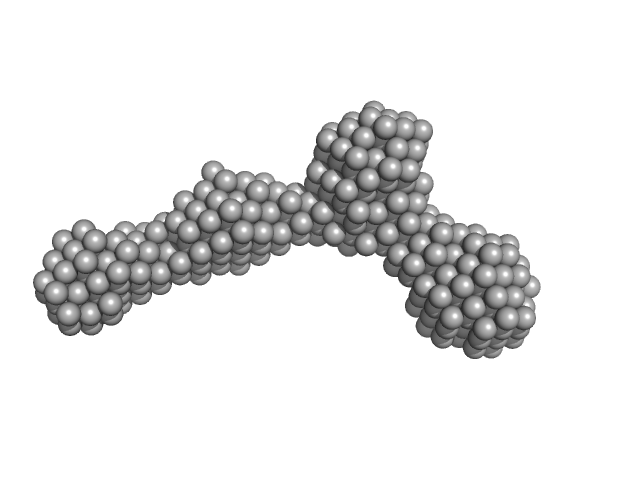
|
| Sample: |
UvrABC system protein B dimer, 156 kDa Mycobacterium tuberculosis (strain … protein
|
| Buffer: |
50 mM Tris pH-8, 200 mM NaCl, 2% glycerol, pH: |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpace, CSIR-Central Drug Research Institute on 2023 Jun 6
|
Inhibition of Mycobacterium tuberculosis UvrB by small molecules: Potent NER disruption and structural insights into dimer conformation.
Int J Biol Macromol :147338 (2025)
Jahan F, Anand S, Kumar S, Pandey G, Siddiqi MI, Krishnan MY, Ramachandran R
|
| RgGuinier |
6.7 |
nm |
| Dmax |
17.5 |
nm |
| VolumePorod |
120 |
nm3 |
|
|
UniProt ID: P01024 (1-1663) Complement C3 (Δ668-671)
|
|
|
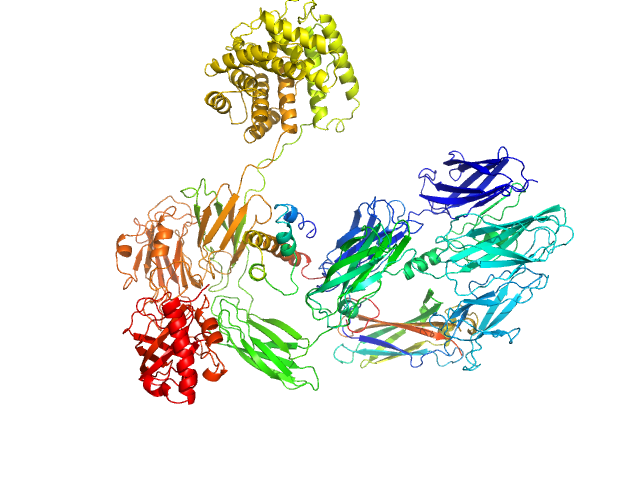
|
| Sample: |
Complement C3 (Δ668-671) monomer, 187 kDa Homo sapiens protein
|
| Buffer: |
20 mM MES pH 6.0, 200 mM NaCl, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2022 Nov 7
|
Cryo-EM analysis of complement C3 reveals a reversible major opening of the macroglobulin ring.
Nat Struct Mol Biol (2025)
Gadeberg TAF, Jørgensen MH, Olesen HG, Lorentzen J, Harwood SL, Almeida AV, Fruergaard MU, Jensen RK, Kanis P, Pedersen H, Tranchant E, Petersen SV, Thøgersen IB, Kragelund BB, Lyons JA, Enghild JJ, Andersen GR
|
| RgGuinier |
5.4 |
nm |
| Dmax |
21.8 |
nm |
| VolumePorod |
357 |
nm3 |
|
|
UniProt ID: E9Q9C6 (27-2583) Fc fragment of IgG binding protein
|
|
|
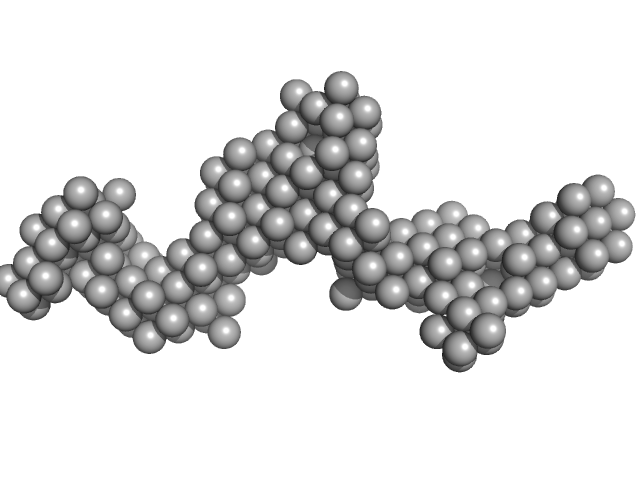
|
| Sample: |
Fc fragment of IgG binding protein dimer, 552 kDa Mus musculus protein
|
| Buffer: |
25 mM HEPES, 100 mM NaCl, 10 mM CaCl2, pH: 7.4 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2020 Nov 20
|
The structure of FCGBP is formed as a disulfide-mediated homodimer between its C-terminal domains.
FEBS J (2025)
Ehrencrona E, Gallego P, Trillo-Muyo S, Garcia-Bonete MJ, Recktenwald CV, Hansson GC, Johansson MEV
|
| RgGuinier |
10.0 |
nm |
| Dmax |
42.0 |
nm |
| VolumePorod |
1462 |
nm3 |
|
|