|
|
|
|
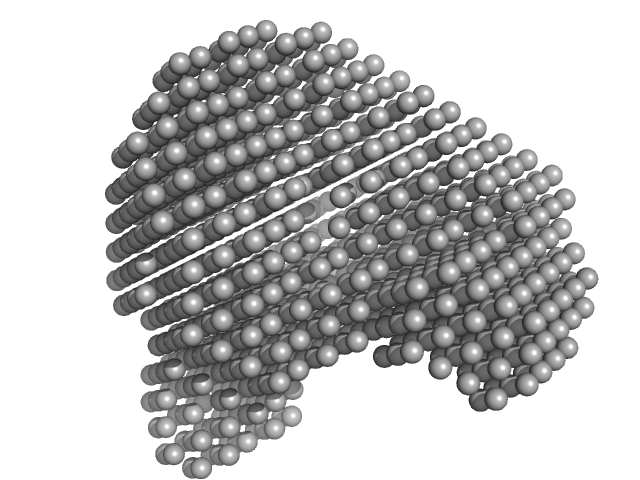
|
| Sample: |
Human serum albumin monomer monomer, 66 kDa Homo sapiens protein
|
| Buffer: |
50 mM HEPES, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2014 Jan 22
|
Correlation Map, a goodness-of-fit test for one-dimensional X-ray scattering spectra.
Nat Methods 12(5):419-22 (2015)
Franke D, Jeffries CM, Svergun DI
|
| RgGuinier |
2.8 |
nm |
| Dmax |
8.4 |
nm |
| VolumePorod |
103 |
nm3 |
|
|
|
|
|
|
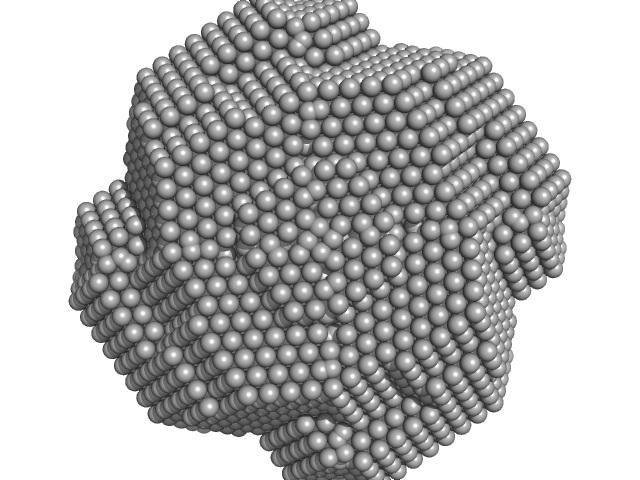
|
| Sample: |
Xylose Isomerase tetramer, 173 kDa Streptomyces rubiginosus protein
|
| Buffer: |
20 mM HEPES 200 mM Na2SO4 50 mM K2SO4 500 % v/v D2O 1 mM MgCl2, pH: 6.6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Dec 10
|
Correlation Map, a goodness-of-fit test for one-dimensional X-ray scattering spectra.
Nat Methods 12(5):419-22 (2015)
Franke D, Jeffries CM, Svergun DI
|
| RgGuinier |
3.2 |
nm |
| Dmax |
9.1 |
nm |
| VolumePorod |
234 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Bacterial chalcone isomerase hexamer, 194 kDa Eubacterium ramulus protein
|
| Buffer: |
50 mM sodium phosphate, pH: 6.8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase.
Acta Crystallogr D Biol Crystallogr 71(Pt 4):907-17 (2015)
Thomsen M, Tuukkanen A, Dickerhoff J, Palm GJ, Kratzat H, Svergun DI, Weisz K, Bornscheuer UT, Hinrichs W
|
| RgGuinier |
4.0 |
nm |
| Dmax |
13.0 |
nm |
| VolumePorod |
320 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Chalcone isomerase with Naringenin hexamer, 194 kDa Eubacterium ramulus protein
|
| Buffer: |
50 mM sodium phosphate, pH: 6.8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase.
Acta Crystallogr D Biol Crystallogr 71(Pt 4):907-17 (2015)
Thomsen M, Tuukkanen A, Dickerhoff J, Palm GJ, Kratzat H, Svergun DI, Weisz K, Bornscheuer UT, Hinrichs W
|
| RgGuinier |
3.7 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
320 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Chalcone isomerase deltaLid hexamer, 181 kDa Eubacterium ramulus protein
|
| Buffer: |
50 mM sodium phosphate, pH: 6.8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
Structure and catalytic mechanism of the evolutionarily unique bacterial chalcone isomerase.
Acta Crystallogr D Biol Crystallogr 71(Pt 4):907-17 (2015)
Thomsen M, Tuukkanen A, Dickerhoff J, Palm GJ, Kratzat H, Svergun DI, Weisz K, Bornscheuer UT, Hinrichs W
|
| RgGuinier |
3.6 |
nm |
| Dmax |
11.0 |
nm |
| VolumePorod |
270 |
nm3 |
|
|
|
|
|
|
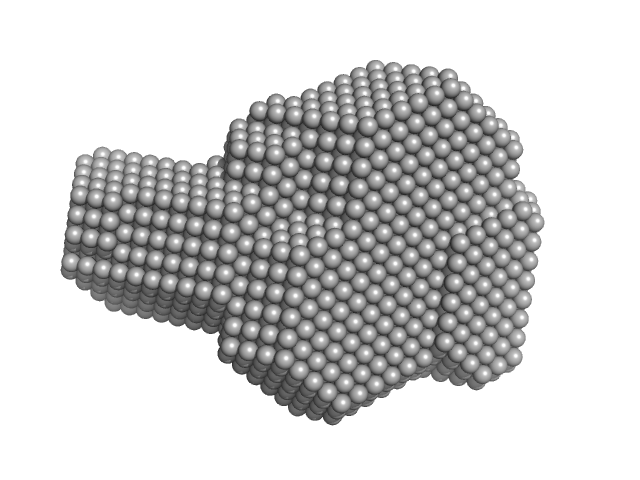
|
| Sample: |
Integrin beta-4 monomer, 23 kDa Homo sapiens protein
|
| Buffer: |
20 mM Sodium Phosphate 150 mM NaCl 5% glycerol 3 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Aug 6
|
Combination of X-ray crystallography, SAXS and DEER to obtain the structure of the FnIII-3,4 domains of integrin α6β4.
Acta Crystallogr D Biol Crystallogr 71(Pt 4):969-85 (2015)
Alonso-García N, García-Rubio I, Manso JA, Buey RM, Urien H, Sonnenberg A, Jeschke G, de Pereda JM
|
| RgGuinier |
2.2 |
nm |
| Dmax |
7.0 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|
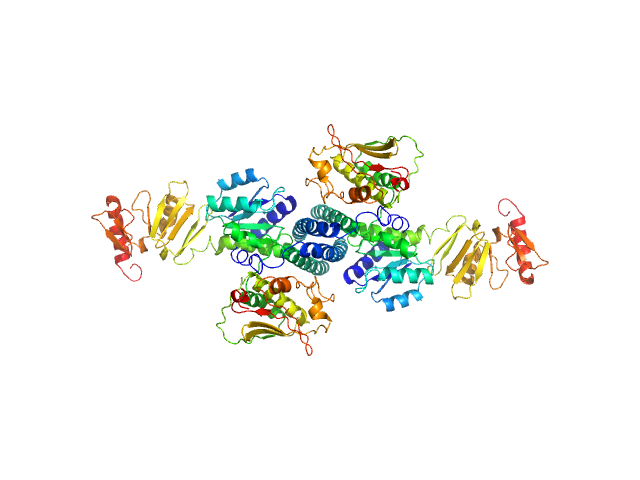
|
| Sample: |
Histidine protein kinase dimer, 54 kDa Streptococcus pneumoniae protein
Response regulator dimer, 61 kDa Streptococcus pneumoniae protein
|
| Buffer: |
20 mM Tris 200 mM NaCl 5% (v/v) Glycerol 5 mM β-mercaptoethanol, pH: 7.5 |
| Experiment: |
SAXS
data collected at Bruker Nanostar, IBBMC on 2012 May 16
|
Modeling the ComD/ComE/comcde interaction network using small angle X-ray scattering.
FEBS J 282(8):1538-53 (2015)
Sanchez D, Boudes M, van Tilbeurgh H, Durand D, Quevillon-Cheruel S
|
| RgGuinier |
4.0 |
nm |
| Dmax |
16.0 |
nm |
| VolumePorod |
175 |
nm3 |
|
|
|
|
|
|
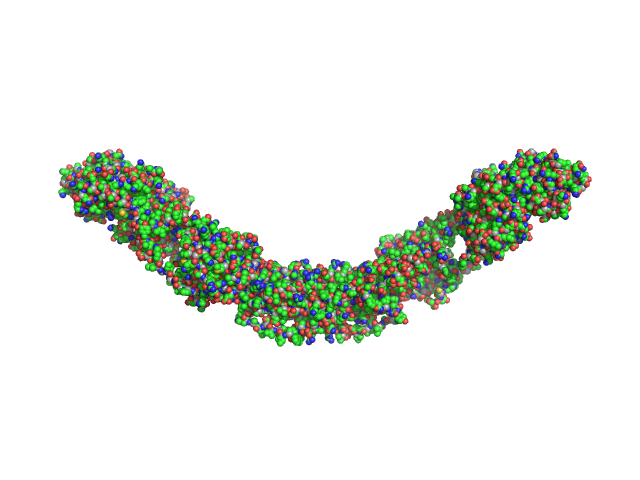
|
| Sample: |
Maltose Binding Protein fused to Protein Interacting with C kinase 1 dimer, 174 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris 300 mM NaCl 1 mM maltose 1 mM EGTA 2 mM DTT, pH: 7.5 |
| Experiment: |
SAXS
data collected at G1, Cornell High Energy Synchrotron Source (CHESS) on 2015 Oct 13
|
PICK1 is implicated in organelle motility in an Arp2/3 complex-independent manner.
Mol Biol Cell 26(7):1308-22 (2015)
Madasu Y, Yang C, Boczkowska M, Bethoney KA, Zwolak A, Rebowski G, Svitkina T, Dominguez R
|
| RgGuinier |
8.4 |
nm |
| Dmax |
27.6 |
nm |
| VolumePorod |
483 |
nm3 |
|
|
|
|
|
|
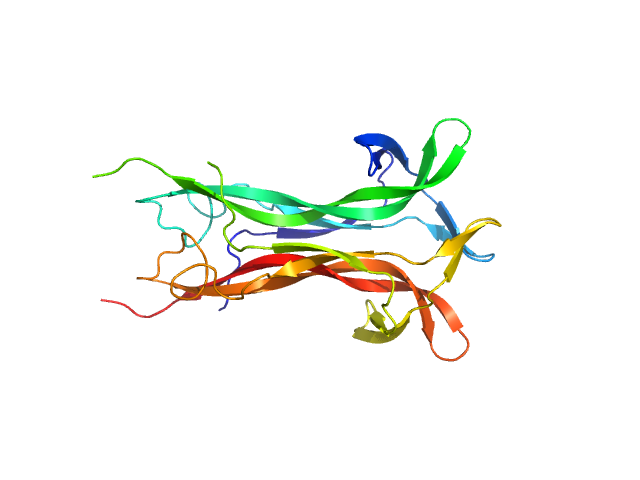
|
| Sample: |
Beta-nerve growth factor dimer, 24 kDa Homo sapiens protein
|
| Buffer: |
50 mM Na-phosphate, 1 mM EDTA, pH: 7 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jul 2
|
The conundrum of the high-affinity NGF binding site formation unveiled?
Biophys J 108(3):687-97 (2015)
Covaceuszach S, Konarev PV, Cassetta A, Paoletti F, Svergun DI, Lamba D, Cattaneo A
|
|
|
|
|
|
|
|
| Sample: |
Ethylene Receptor 1 dimer, 129 kDa Arabidopsis thaliana protein
|
| Buffer: |
20 mM Tris-NDSB 150 mM NaCl 1mM DTT 250 mM NDSB, pH: 8.8 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2011 Mar 19
|
Structural model of the cytosolic domain of the plant ethylene receptor 1 (ETR1).
J Biol Chem 290(5):2644-58 (2015)
Mayerhofer H, Panneerselvam S, Kaljunen H, Tuukkanen A, Mertens HD, Mueller-Dieckmann J
|
| RgGuinier |
4.7 |
nm |
| Dmax |
15.8 |
nm |
| VolumePorod |
316 |
nm3 |
|
|