|
|
|
|
|
| Sample: |
Prosaposin (Saposin-A) dimer, 19 kDa Homo sapiens protein
2,2-didecylpropane-1,3-bis-β-D-maltopyranoside 0, 15 kDa
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at 12-ID-B SAXS/WAXS, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 18
|
Stable Picodisc Assemblies from Saposin Proteins and Branched Detergents.
Biochemistry 60(14):1108-1119 (2021)
Kurgan KW, Chen B, Brown KA, Falco Cobra P, Ye X, Ge Y, Gellman SH
|
| RgGuinier |
2.5 |
nm |
| Dmax |
7.0 |
nm |
|
|
|
|
|
|
|
| Sample: |
Prosaposin (Saposin-B, R232W mutant) dimer, 18 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at 12-ID-B SAXS/WAXS, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 18
|
Stable Picodisc Assemblies from Saposin Proteins and Branched Detergents.
Biochemistry 60(14):1108-1119 (2021)
Kurgan KW, Chen B, Brown KA, Falco Cobra P, Ye X, Ge Y, Gellman SH
|
| RgGuinier |
2.0 |
nm |
| Dmax |
5.8 |
nm |
|
|
|
|
|
|
|
| Sample: |
1,2,5,6-tetra-β-D-glucopyranoside-3,4-O-Di-dodecyl-d-mannitol decamer, 12 kDa
Prosaposin (Saposin-B, R232W mutant) dimer, 18 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at 12-ID-B SAXS/WAXS, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 18
|
Stable Picodisc Assemblies from Saposin Proteins and Branched Detergents.
Biochemistry 60(14):1108-1119 (2021)
Kurgan KW, Chen B, Brown KA, Falco Cobra P, Ye X, Ge Y, Gellman SH
|
| RgGuinier |
2.3 |
nm |
| Dmax |
6.9 |
nm |
|
|
|
|
|
|
|
| Sample: |
1,2,5,6-tetra-β-D-glucopyranoside-3,4-O-Di-tridecyl-d-mannitol decamer, 12 kDa
Prosaposin (Saposin-B, R232W mutant) dimer, 18 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at 12-ID-B SAXS/WAXS, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 18
|
Stable Picodisc Assemblies from Saposin Proteins and Branched Detergents.
Biochemistry 60(14):1108-1119 (2021)
Kurgan KW, Chen B, Brown KA, Falco Cobra P, Ye X, Ge Y, Gellman SH
|
| RgGuinier |
2.6 |
nm |
| Dmax |
7.7 |
nm |
|
|
|
|
|
|
|
| Sample: |
2,2-didecylpropane-1,3-bis-β-D-maltopyranoside 0, 15 kDa
Prosaposin (Saposin-B, R232W mutant) dimer, 18 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at 12-ID-B SAXS/WAXS, Advanced Photon Source (APS), Argonne National Laboratory on 2020 Feb 18
|
Stable Picodisc Assemblies from Saposin Proteins and Branched Detergents.
Biochemistry 60(14):1108-1119 (2021)
Kurgan KW, Chen B, Brown KA, Falco Cobra P, Ye X, Ge Y, Gellman SH
|
| RgGuinier |
2.8 |
nm |
| Dmax |
8.9 |
nm |
|
|
|
|
|
|
|
| Sample: |
Von Willebrand factor monomer, 11 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2019 Aug 24
|
An Integrative Structural Biology Analysis of Von Willebrand Factor Binding and Processing by ADAMTS-13 in Solution.
J Mol Biol 433(13):166954 (2021)
Del Amo-Maestro L, Sagar A, Pompach P, Goulas T, Scavenius C, Ferrero DS, Castrillo-Briceño M, Taulés M, Enghild JJ, Bernadó P, Gomis-Rüth FX
|
| RgGuinier |
3.1 |
nm |
| Dmax |
14.0 |
nm |
| VolumePorod |
28 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Von Willebrand factor monomer, 11 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2019 Aug 24
|
An Integrative Structural Biology Analysis of Von Willebrand Factor Binding and Processing by ADAMTS-13 in Solution.
J Mol Biol 433(13):166954 (2021)
Del Amo-Maestro L, Sagar A, Pompach P, Goulas T, Scavenius C, Ferrero DS, Castrillo-Briceño M, Taulés M, Enghild JJ, Bernadó P, Gomis-Rüth FX
|
| RgGuinier |
3.0 |
nm |
| Dmax |
14.9 |
nm |
| VolumePorod |
27 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Von Willebrand factor monomer, 11 kDa Homo sapiens protein
A disintegrin and metalloproteinase with thrombospondin motifs 13 monomer, 70 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2019 Aug 24
|
An Integrative Structural Biology Analysis of Von Willebrand Factor Binding and Processing by ADAMTS-13 in Solution.
J Mol Biol 433(13):166954 (2021)
Del Amo-Maestro L, Sagar A, Pompach P, Goulas T, Scavenius C, Ferrero DS, Castrillo-Briceño M, Taulés M, Enghild JJ, Bernadó P, Gomis-Rüth FX
|
| RgGuinier |
4.4 |
nm |
| Dmax |
16.3 |
nm |
| VolumePorod |
170 |
nm3 |
|
|
|
|
|
|
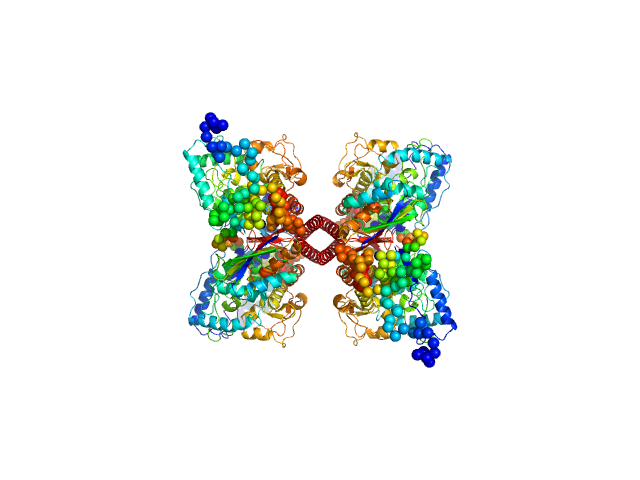
|
| Sample: |
Phenylalanine-4-hydroxylase tetramer, 223 kDa Homo sapiens protein
|
| Buffer: |
0.02 mM Hepes, 0.2 M NaCl,, pH: 7 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Feb 25
|
Modulation of Human Phenylalanine Hydroxylase by 3-Hydroxyquinolin-2(1H)-One Derivatives
Biomolecules 11(3):462 (2021)
Lopes R, Tomé C, Russo R, Paterna R, Leandro J, Candeias N, Gonçalves L, Teixeira M, Sousa P, Guedes R, Vicente J, Gois P, Leandro P
|
| RgGuinier |
4.3 |
nm |
| Dmax |
14.8 |
nm |
| VolumePorod |
336 |
nm3 |
|
|
|
|
|
|
|
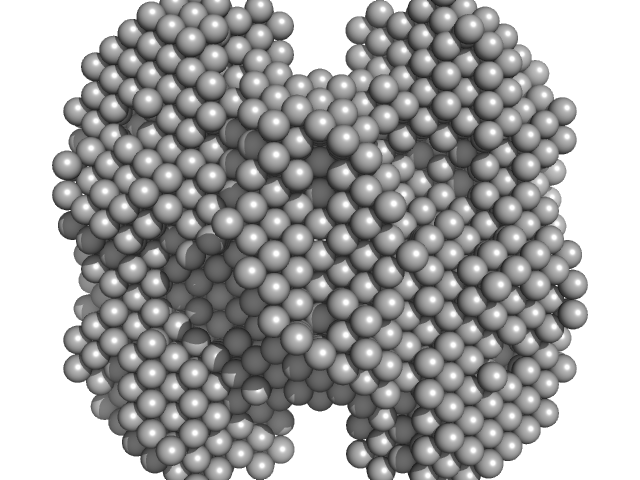
| Sample: |
Phenylalanine-4-hydroxylase tetramer, 223 kDa Homo sapiens protein
|
| Buffer: |
0.02 mM Hepes, 0.2 M NaCl,, pH: 7 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Feb 25
|
Modulation of Human Phenylalanine Hydroxylase by 3-Hydroxyquinolin-2(1H)-One Derivatives
Biomolecules 11(3):462 (2021)
Lopes R, Tomé C, Russo R, Paterna R, Leandro J, Candeias N, Gonçalves L, Teixeira M, Sousa P, Guedes R, Vicente J, Gois P, Leandro P
|
| RgGuinier |
4.0 |
nm |
| Dmax |
12.3 |
nm |
| VolumePorod |
367 |
nm3 |
|
|