|
|
|
|
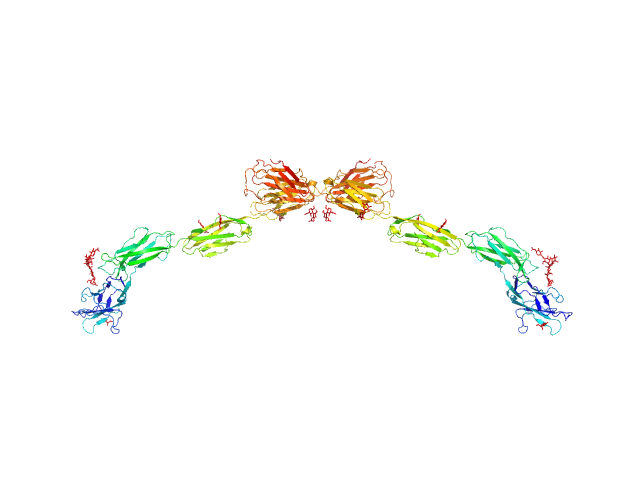
|
| Sample: |
Myelin-associated glycoprotein (20-508; N406Q mutant) monomer, 54 kDa Mus musculus protein
|
| Buffer: |
20 mM HEPES 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2016 Feb 5
|
Structural basis of myelin-associated glycoprotein adhesion and signalling.
Nat Commun 7:13584 (2016)
Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ
|
| RgGuinier |
7.3 |
nm |
| Dmax |
25.6 |
nm |
| VolumePorod |
193 |
nm3 |
|
|
|
|
|
|
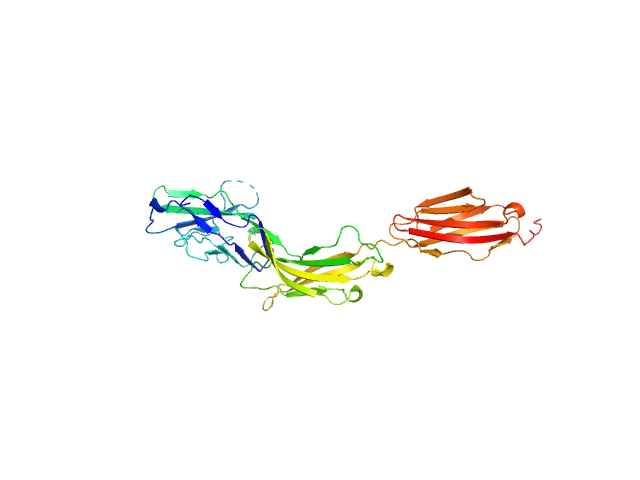
|
| Sample: |
Myelin-associated glycoprotein Ig domains 1-3 monomer, 35 kDa Mus musculus protein
|
| Buffer: |
20 mM HEPES 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2014 Sep 11
|
Structural basis of myelin-associated glycoprotein adhesion and signalling.
Nat Commun 7:13584 (2016)
Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ
|
| RgGuinier |
3.9 |
nm |
| Dmax |
13.0 |
nm |
| VolumePorod |
59 |
nm3 |
|
|
|
|
|
|
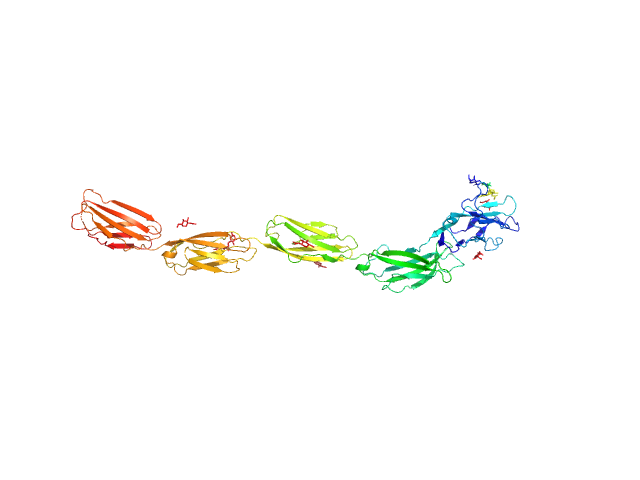
|
| Sample: |
Myelin-associated glycoprotein (20-508; I473E mutant) monomer, 54 kDa Mus musculus protein
|
| Buffer: |
20 mM HEPES 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2014 Sep 11
|
Structural basis of myelin-associated glycoprotein adhesion and signalling.
Nat Commun 7:13584 (2016)
Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ
|
| RgGuinier |
6.0 |
nm |
| Dmax |
21.2 |
nm |
| VolumePorod |
100 |
nm3 |
|
|
|
|
|
|
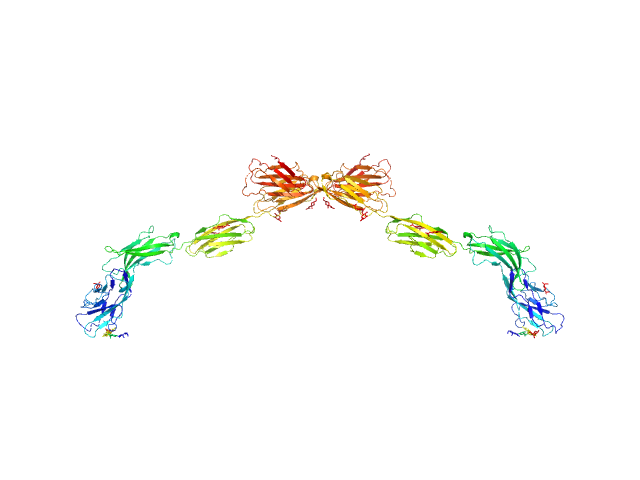
|
| Sample: |
Myelin-associated glycoprotein (20-508; N406Q mutant) monomer, 54 kDa Mus musculus protein
|
| Buffer: |
20 mM HEPES 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2015 Jul 28
|
Structural basis of myelin-associated glycoprotein adhesion and signalling.
Nat Commun 7:13584 (2016)
Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ
|
| RgGuinier |
7.8 |
nm |
| Dmax |
29.0 |
nm |
| VolumePorod |
216 |
nm3 |
|
|
|
|
|
|
|
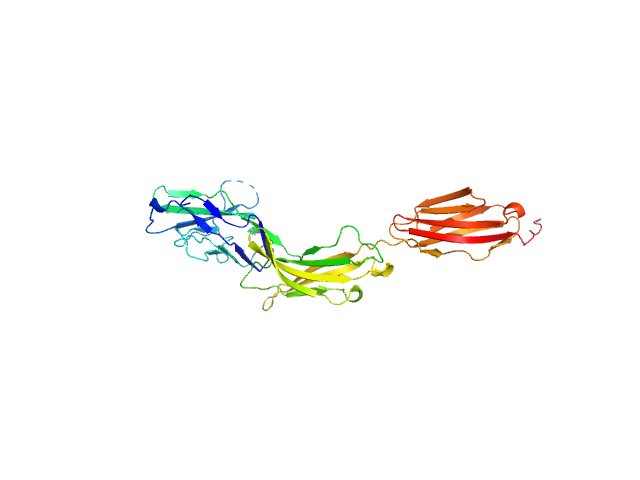
| Sample: |
Myelin-associated glycoprotein Ig domains 1-3 monomer, 35 kDa Mus musculus protein
|
| Buffer: |
20 mM HEPES 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2014 Sep 11
|
Structural basis of myelin-associated glycoprotein adhesion and signalling.
Nat Commun 7:13584 (2016)
Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ
|
| RgGuinier |
3.9 |
nm |
| Dmax |
12.6 |
nm |
| VolumePorod |
49 |
nm3 |
|
|
|
|
|
|
|
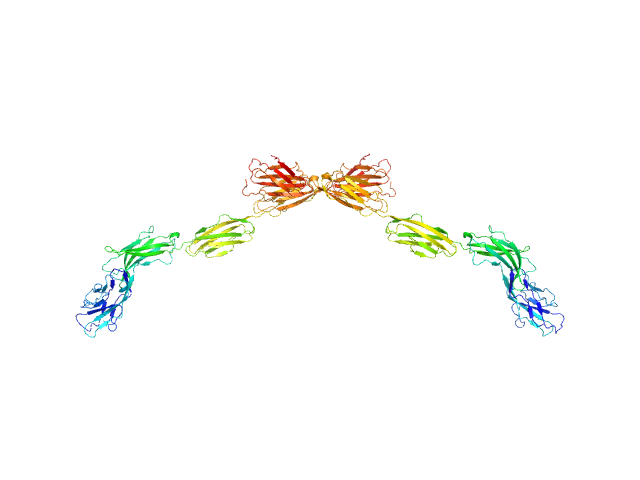
| Sample: |
Myelin-associated glycoprotein Ig domains 1-5 dimer, 108 kDa Mus musculus protein
|
| Buffer: |
20 mM HEPES 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2014 Sep 11
|
Structural basis of myelin-associated glycoprotein adhesion and signalling.
Nat Commun 7:13584 (2016)
Pronker MF, Lemstra S, Snijder J, Heck AJ, Thies-Weesie DM, Pasterkamp RJ, Janssen BJ
|
| RgGuinier |
7.3 |
nm |
| Dmax |
25.5 |
nm |
| VolumePorod |
166 |
nm3 |
|
|
|
|
|
|
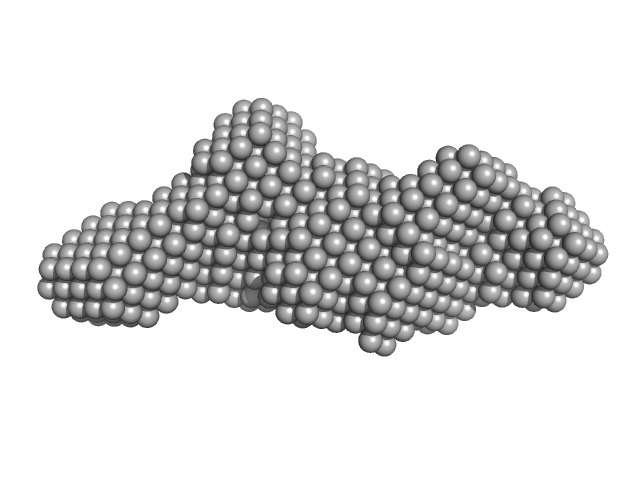
|
| Sample: |
Mouse Leucine-rich repeat transmembrane neuronal protein 2, cLRRTM2 (stability engineered construct) monomer, 40 kDa Mus musculus protein
|
| Buffer: |
20 mM Tris 150 mM NaCl 3% glycerol, pH: 7.4 |
| Experiment: |
SAXS
data collected at ID14-3, ESRF on 2015 Sep 27
|
Crystal Structure of an Engineered LRRTM2 Synaptic Adhesion Molecule and a Model for Neurexin Binding.
Biochemistry 55(6):914-26 (2016)
Paatero A, Rosti K, Shkumatov AV, Sele C, Brunello C, Kysenius K, Singha P, Jokinen V, Huttunen H, Kajander T
|
| RgGuinier |
3.3 |
nm |
| Dmax |
13.1 |
nm |
|
|
|
|
|
|
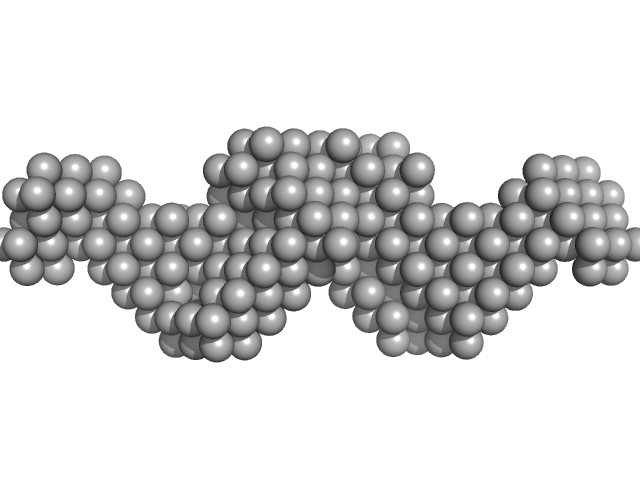
|
| Sample: |
Mouse Leucine-rich repeat transmembrane neuronal protein 2, LRRTM2 dimer, 80 kDa Mus musculus protein
|
| Buffer: |
20 mM Tris 150 mM NaCl 3% glycerol, pH: 7.4 |
| Experiment: |
SAXS
data collected at ID14-3, ESRF on 2015 Jun 28
|
Crystal Structure of an Engineered LRRTM2 Synaptic Adhesion Molecule and a Model for Neurexin Binding.
Biochemistry 55(6):914-26 (2016)
Paatero A, Rosti K, Shkumatov AV, Sele C, Brunello C, Kysenius K, Singha P, Jokinen V, Huttunen H, Kajander T
|
| RgGuinier |
4.2 |
nm |
| Dmax |
21.6 |
nm |
|
|
|
|
|
|
|
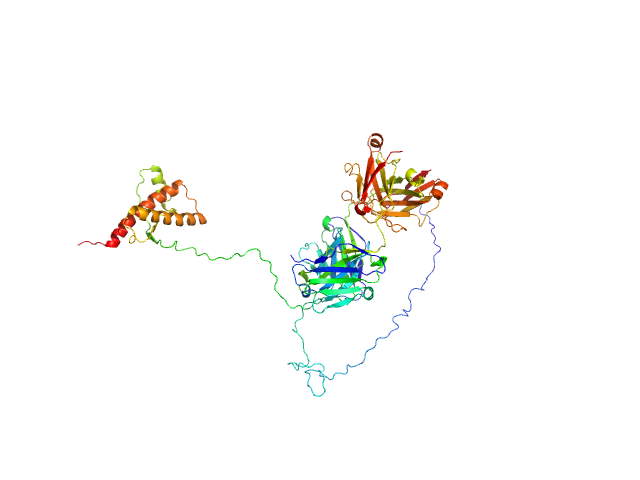
| Sample: |
Major prion protein monomer, 23 kDa Mus musculus protein
P-Clone Fab, Chimera monomer, 47 kDa Homo sapiens protein
|
| Buffer: |
sodium acetate buffer (20 mM sodium acetate, pH 5.1; 150 mM NaCl), pH: 5.1 |
| Experiment: |
SAXS
data collected at BL4-2, Stanford Synchrotron Radiation Lightsource (SSRL) on 2013 Dec 5
|
Prion Protein-Antibody Complexes Characterized by Chromatography-Coupled Small-Angle X-Ray Scattering.
Biophys J 109(4):793-805 (2015)
Carter L, Kim SJ, Schneidman-Duhovny D, Stöhr J, Poncet-Montange G, Weiss TM, Tsuruta H, Prusiner SB, Sali A
|
| RgGuinier |
3.9 |
nm |
| Dmax |
14.5 |
nm |
| VolumePorod |
106 |
nm3 |
|
|
|
|
|
|
|
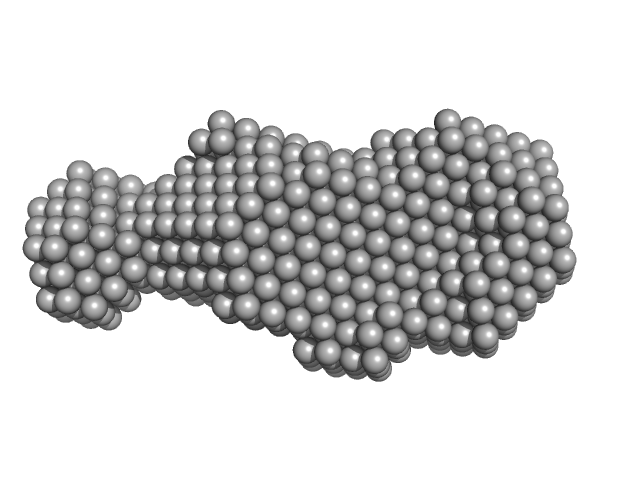
| Sample: |
Hyaluronate binding domain of CD44 antigen monomer, 18 kDa Homo sapiens protein
Single-chain Variable Fragment of Antibody MEM-85 monomer, 29 kDa Mus musculus protein
|
| Buffer: |
PBS, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Oct 31
|
Molecular mechanism for the action of the anti-CD44 monoclonal antibody MEM-85.
J Struct Biol 191(2):214-23 (2015)
Škerlová J, Král V, Kachala M, Fábry M, Bumba L, Svergun DI, Tošner Z, Veverka V, Řezáčová P
|
| RgGuinier |
2.7 |
nm |
| Dmax |
9.4 |
nm |
| VolumePorod |
57 |
nm3 |
|
|