|
|
|
|
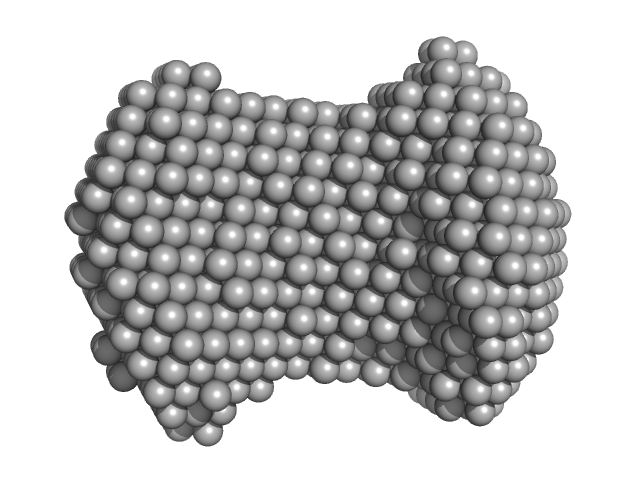
|
| Sample: |
G-quadrupex monomer, 6 kDa Hepatitis B virus DNA
|
| Buffer: |
20 mM HEPES, 100 mM KCl, 1 mM EDTA, pH: 7.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2018 Jan 15
|
G-quadruplex from HBV genome
Trushar Patel
|
| RgGuinier |
1.7 |
nm |
| Dmax |
4.0 |
nm |
| VolumePorod |
13 |
nm3 |
|
|
|
|
|
|
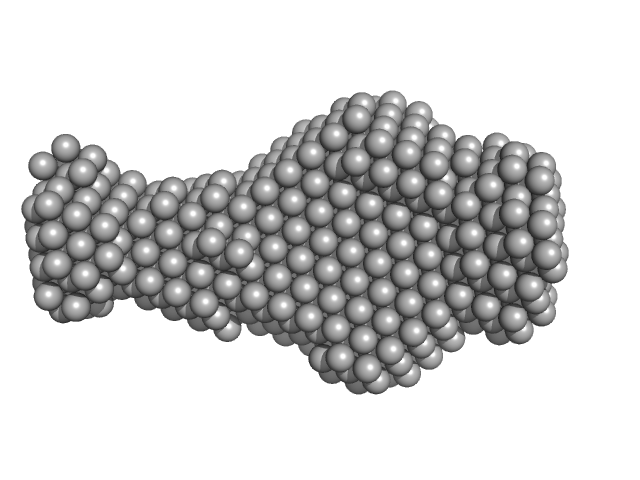
|
| Sample: |
G-quadruplex mutant monomer, 6 kDa Hepatitis B virus DNA
|
| Buffer: |
20 mM HEPES, 100 mM KCl, 1 mM EDTA, pH: 7.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2018 Jan 15
|
G-quadruplex from HBV genome
Trushar Patel
|
| RgGuinier |
1.7 |
nm |
| Dmax |
5.5 |
nm |
| VolumePorod |
11 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
E3 ubiquitin/ISG15 ligase TRIM25 dimer, 100 kDa Homo sapiens protein
|
| Buffer: |
20 mM MES, 75 mM NaCl, 1 mM TCEP, pH: 6.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2019 Nov 19
|
The molecular dissection of TRIM25's RNA-binding mechanism provides key insights into its antiviral activity.
Nat Commun 15(1):8485 (2024)
Álvarez L, Haubrich K, Iselin L, Gillioz L, Ruscica V, Lapouge K, Augsten S, Huppertz I, Choudhury NR, Simon B, Masiewicz P, Lethier M, Cusack S, Rittinger K, Gabel F, Leitner A, Michlewski G, Hentze ...
|
| RgGuinier |
6.8 |
nm |
| Dmax |
30.2 |
nm |
|
|
|
|
|
|
|
| Sample: |
E3 ubiquitin/ISG15 ligase TRIM25 dimer, 100 kDa Homo sapiens protein
pre-let-7-a-1@1 monomer, 9 kDa synthetic construct RNA
|
| Buffer: |
20 mM MES, 75 mM NaCl, 1 mM TCEP, pH: 6.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2019 May 14
|
The molecular dissection of TRIM25's RNA-binding mechanism provides key insights into its antiviral activity.
Nat Commun 15(1):8485 (2024)
Álvarez L, Haubrich K, Iselin L, Gillioz L, Ruscica V, Lapouge K, Augsten S, Huppertz I, Choudhury NR, Simon B, Masiewicz P, Lethier M, Cusack S, Rittinger K, Gabel F, Leitner A, Michlewski G, Hentze ...
|
| RgGuinier |
5.7 |
nm |
| Dmax |
19.8 |
nm |
|
|
|
|
|
|
|
| Sample: |
E3 ubiquitin/ISG15 ligase TRIM25 dimer, 100 kDa Homo sapiens protein
pre-let-7-a-1@1 monomer, 9 kDa synthetic construct RNA
|
| Buffer: |
20 mM MES, 75 mM NaCl, 1 mM TCEP, pH: 6.5
|
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Jun 4
|
The molecular dissection of TRIM25's RNA-binding mechanism provides key insights into its antiviral activity.
Nat Commun 15(1):8485 (2024)
Álvarez L, Haubrich K, Iselin L, Gillioz L, Ruscica V, Lapouge K, Augsten S, Huppertz I, Choudhury NR, Simon B, Masiewicz P, Lethier M, Cusack S, Rittinger K, Gabel F, Leitner A, Michlewski G, Hentze ...
|
| RgGuinier |
5.7 |
nm |
| Dmax |
23.0 |
nm |
|
|
|
|
|
|
|
| Sample: |
E3 ubiquitin/ISG15 ligase TRIM25 dimer, 100 kDa Homo sapiens protein
lnczc3h7a_304-326 monomer, 8 kDa Homo sapiens RNA
|
| Buffer: |
20 mM MES, 75 mM NaCl, 1 mM TCEP, pH: 6.5
|
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2020 Jun 12
|
The molecular dissection of TRIM25's RNA-binding mechanism provides key insights into its antiviral activity.
Nat Commun 15(1):8485 (2024)
Álvarez L, Haubrich K, Iselin L, Gillioz L, Ruscica V, Lapouge K, Augsten S, Huppertz I, Choudhury NR, Simon B, Masiewicz P, Lethier M, Cusack S, Rittinger K, Gabel F, Leitner A, Michlewski G, Hentze ...
|
| RgGuinier |
5.8 |
nm |
| Dmax |
16.6 |
nm |
|
|
|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.2 |
nm |
| Dmax |
6.9 |
nm |
| VolumePorod |
49 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
High affinity immunoglobulin epsilon receptor subunit gamma monomer, 2 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.0 |
nm |
| VolumePorod |
54 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
T-cell surface glycoprotein CD3 gamma chain monomer, 3 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.1 |
nm |
| VolumePorod |
56 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Tyrosine-protein kinase SYK monomer, 30 kDa Homo sapiens protein
TYRO protein tyrosine kinase-binding protein monomer, 2 kDa Homo sapiens protein
|
| Buffer: |
10 mM HEPES, 150 mM NaCl, 1 mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2023 Nov 13
|
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures
Structure (2024)
Bradshaw W, Harris G, Gileadi O, Katis V
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.1 |
nm |
| VolumePorod |
53 |
nm3 |
|
|