|
|
|
|
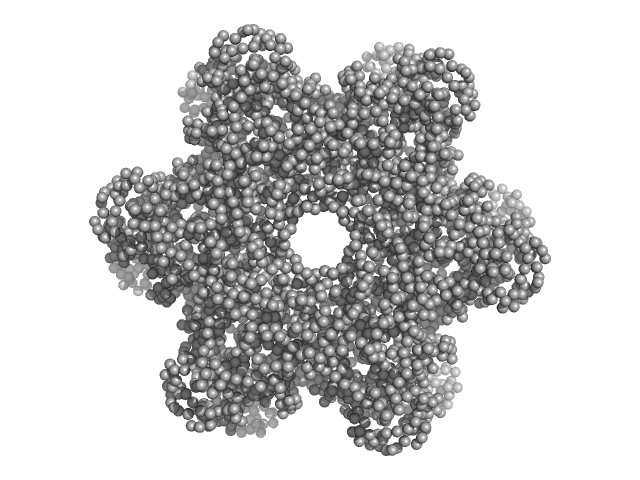
|
| Sample: |
Protein DPCD trimer, 71 kDa Homo sapiens protein
RuvB-like 1 trimer, 155 kDa Homo sapiens protein
RuvB-like 2 trimer, 156 kDa Homo sapiens protein
|
| Buffer: |
20 mM HEPES, 150 mM NaCl, 1% glycerol, 5 mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at SWING, SOLEIL on 2019 Mar 21
|
Deciphering cellular and molecular determinants of human DPCD protein in complex with RUVBL1/RUVBL2 AAA-ATPases.
J Mol Biol :167760 (2022)
Dos Santos Morais R, Santo PE, Ley M, Schelcher C, Abel Y, Plassart L, Deslignière E, Chagot ME, Quinternet M, Paiva ACF, Hessmann S, Morellet N, M F Sousa P, Vandermoere F, Bertrand E, Charpentier B,...
|
| RgGuinier |
5.4 |
nm |
| Dmax |
15.9 |
nm |
| VolumePorod |
856 |
nm3 |
|