UniProt ID: P19491 (None-None) Glutamate receptor 2
|
|
|
|
| Sample: |
Glutamate receptor 2 monomer, 368 kDa Rattus norvegicus protein
|
| Buffer: |
D2O based buffer. 20 mM Tris/DCl, 100 mM NaCl, 0.5 mM deuterated n-dodecyl-β-D-maltopyranoside (synthesized to match out at 100% D2O), pH: 7.5 |
| Experiment: |
SANS
data collected at KWS1, FRM2 on 2017 Sep 19
|
Small-angle neutron scattering studies on the AMPA receptor GluA2 in the resting, AMPA-bound and GYKI-53655-bound states.
IUCrJ 5(Pt 6):780-793 (2018)
Larsen AH, Dorosz J, Thorsen TS, Johansen NT, Darwish T, Midtgaard SR, Arleth L, Kastrup JS
|
| RgGuinier |
6.0 |
nm |
| Dmax |
17.9 |
nm |
| VolumePorod |
396 |
nm3 |
|
|
UniProt ID: P19491 (None-None) Glutamate receptor 2
|
|
|
|
| Sample: |
Glutamate receptor 2 monomer, 368 kDa Rattus norvegicus protein
|
| Buffer: |
D2O based buffer. 1 mM AMPA, 20 mM Tris/DCl, 100 mM NaCl, 0.5 mM deuterated n-dodecyl-β-D-maltopyranoside (synthesized to match out at 100% D2O), pH: 7.5 |
| Experiment: |
SANS
data collected at KWS1, FRM2 on 2016 Oct 19
|
Small-angle neutron scattering studies on the AMPA receptor GluA2 in the resting, AMPA-bound and GYKI-53655-bound states.
IUCrJ 5(Pt 6):780-793 (2018)
Larsen AH, Dorosz J, Thorsen TS, Johansen NT, Darwish T, Midtgaard SR, Arleth L, Kastrup JS
|
| RgGuinier |
6.3 |
nm |
| Dmax |
18.4 |
nm |
| VolumePorod |
407 |
nm3 |
|
|
UniProt ID: P19491 (None-None) Glutamate receptor 2
|
|
|
|
| Sample: |
Glutamate receptor 2 monomer, 368 kDa Rattus norvegicus protein
|
| Buffer: |
D2O based buffer. 10 mM AMPA, 20 mM Tris/DCl, 100 mM NaCl, 0.5 mM deuterated n-dodecyl-β-D-maltopyranoside (synthesized to match out at 100% D2O), pH: 5.5 |
| Experiment: |
SANS
data collected at KWS1, FRM2 on 2017 Sep 19
|
Small-angle neutron scattering studies on the AMPA receptor GluA2 in the resting, AMPA-bound and GYKI-53655-bound states.
IUCrJ 5(Pt 6):780-793 (2018)
Larsen AH, Dorosz J, Thorsen TS, Johansen NT, Darwish T, Midtgaard SR, Arleth L, Kastrup JS
|
| RgGuinier |
6.5 |
nm |
| Dmax |
18.9 |
nm |
| VolumePorod |
899 |
nm3 |
|
|
UniProt ID: P19491 (None-None) Glutamate receptor 2
|
|
|
|
| Sample: |
Glutamate receptor 2 monomer, 368 kDa Rattus norvegicus protein
|
| Buffer: |
D2O based buffer. 1 mM GYKI-53655, 20 mM Tris/DCl, 100 mM NaCl, 0.5 mM deuterated n-dodecyl-β-D-maltopyranoside (synthesized to match out at 100% D2O), pH: 7.5 |
| Experiment: |
SANS
data collected at KWS1, FRM2 on 2016 Oct 19
|
Small-angle neutron scattering studies on the AMPA receptor GluA2 in the resting, AMPA-bound and GYKI-53655-bound states.
IUCrJ 5(Pt 6):780-793 (2018)
Larsen AH, Dorosz J, Thorsen TS, Johansen NT, Darwish T, Midtgaard SR, Arleth L, Kastrup JS
|
| RgGuinier |
6.3 |
nm |
| Dmax |
18.6 |
nm |
| VolumePorod |
384 |
nm3 |
|
|
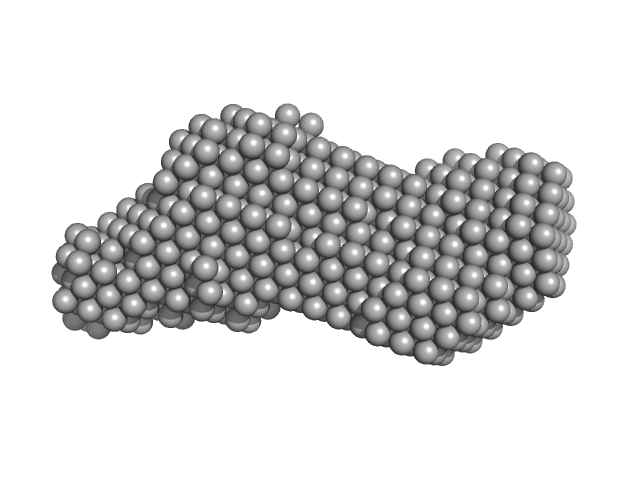
UniProt ID: O00443 (1405-1686) Phox Homology (PX) - C2 domains of human Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha
|
|
|
|
| Sample: |
Phox Homology (PX) - C2 domains of human Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha monomer, 33 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris 200 mM NaCl 5% Glycerol 0.5 mM TCEP, pH: 8.5 |
| Experiment: |
SAXS
data collected at SAXS/WAXS, Australian Synchrotron on 2017 Oct 20
|
Molecular Basis for Membrane Recruitment by the PX and C2 Domains of Class II Phosphoinositide 3-Kinase-C2α.
Structure (2018)
Chen KE, Tillu VA, Chandra M, Collins BM
|
| RgGuinier |
2.6 |
nm |
| Dmax |
9.3 |
nm |
| VolumePorod |
43 |
nm3 |
|
|
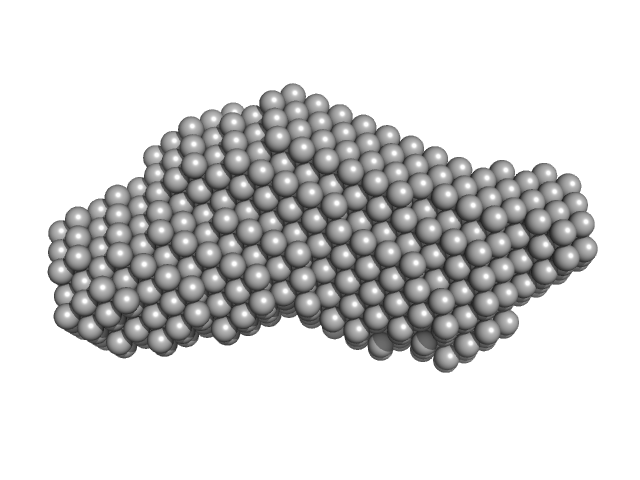
UniProt ID: O00443 (1405-1686) Phox Homology (PX) - C2 domains of human Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha
|
|
|
|
| Sample: |
Phox Homology (PX) - C2 domains of human Phosphatidylinositol 4-phosphate 3-kinase C2 domain-containing subunit alpha monomer, 33 kDa Homo sapiens protein
|
| Buffer: |
25 mM Tris 200 mM NaCl 5% Glycerol 0.5 mM TCEP 4 mM InsP6, pH: 8.5 |
| Experiment: |
SAXS
data collected at SAXS/WAXS, Australian Synchrotron on 2017 Oct 20
|
Molecular Basis for Membrane Recruitment by the PX and C2 Domains of Class II Phosphoinositide 3-Kinase-C2α.
Structure (2018)
Chen KE, Tillu VA, Chandra M, Collins BM
|
| RgGuinier |
2.6 |
nm |
| Dmax |
9.3 |
nm |
| VolumePorod |
48 |
nm3 |
|
|
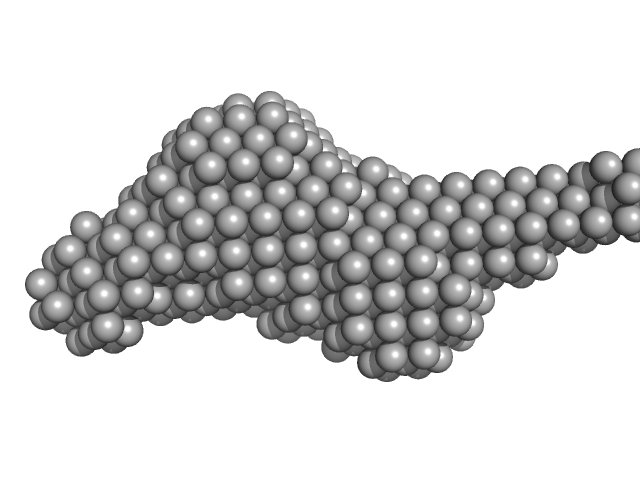
UniProt ID: Q8NBF2 (1-726) NHL repeat-containing protein 2
|
|
|
|
| Sample: |
NHL repeat-containing protein 2 monomer, 80 kDa Homo sapiens protein
|
| Buffer: |
20 mM BisTris, 100 mM NaCl, 2 mM DTT, pH: 6.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2017 Jul 8
|
Structural analysis of human NHLRC2, mutations of which are associated with FINCA disease.
PLoS One 13(8):e0202391 (2018)
Biterova E, Ignatyev A, Uusimaa J, Hinttala R, Ruddock LW
|
| RgGuinier |
3.6 |
nm |
| Dmax |
14.7 |
nm |
| VolumePorod |
121 |
nm3 |
|
|
UniProt ID: O43765 (1-313) Small glutamine-rich tetratricopeptide repeat-containing protein alpha full length
|
|
|
|
| Sample: |
Small glutamine-rich tetratricopeptide repeat-containing protein alpha full length dimer, 68 kDa Homo sapiens protein
|
| Buffer: |
10 mM potassium phosphate, 100 mM NaCl, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 5
|
Structural complexity of the co-chaperone SGTA: a conserved C-terminal region is implicated in dimerization and substrate quality control.
BMC Biol 16(1):76 (2018)
Martínez-Lumbreras S, Krysztofinska EM, Thapaliya A, Spilotros A, Matak-Vinkovic D, Salvadori E, Roboti P, Nyathi Y, Muench JH, Roessler MM, Svergun DI, High S, Isaacson RL
|
|
|
UniProt ID: O43765 (1-213) Small glutamine-rich tetratricopeptide repeat-containing protein alpha Nterminal-TPR domains
|
|
|
|
| Sample: |
Small glutamine-rich tetratricopeptide repeat-containing protein alpha Nterminal-TPR domains dimer, 47 kDa Homo sapiens protein
|
| Buffer: |
10 mM potassium phosphate, 100 mM NaCl, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2015 Jun 5
|
Structural complexity of the co-chaperone SGTA: a conserved C-terminal region is implicated in dimerization and substrate quality control.
BMC Biol 16(1):76 (2018)
Martínez-Lumbreras S, Krysztofinska EM, Thapaliya A, Spilotros A, Matak-Vinkovic D, Salvadori E, Roboti P, Nyathi Y, Muench JH, Roessler MM, Svergun DI, High S, Isaacson RL
|
|
|
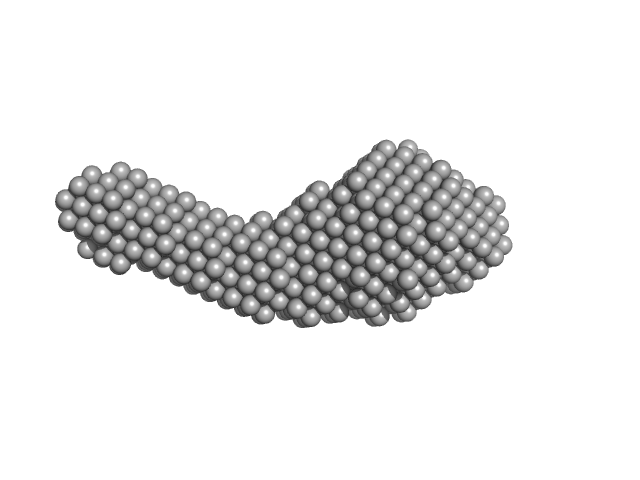
UniProt ID: P32455 (None-None) Guanylate-binding protein 1
|
|
|
|
| Sample: |
Guanylate-binding protein 1 monomer, 68 kDa Homo sapiens protein
|
| Buffer: |
50 mM TRIS, 5 mM MgCl2, 150 mM NaCl, pH: 7.9 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2018 Apr 30
|
Integrative dynamic structural biology unveils conformers essential for the oligomerization of a large GTPase.
Elife 12 (2023)
Peulen TO, Hengstenberg CS, Biehl R, Dimura M, Lorenz C, Valeri A, Folz J, Hanke CA, Ince S, Vöpel T, Farago B, Gohlke H, Klare JP, Stadler AM, Seidel CAM, Herrmann C
|
| RgGuinier |
3.9 |
nm |
| Dmax |
14.4 |
nm |
| VolumePorod |
105 |
nm3 |
|
|