|
|
|
|
|
| Sample: |
Protein sex-lethal monomer, 20 kDa Drosophila melanogaster protein
|
| Buffer: |
10 mM KP, 50 mM NaCl, 10 mM DTT, pH: 6 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2016 Oct 16
|
A General Small-Angle X-ray Scattering-Based Screening Protocol Validated for Protein-RNA Interactions.
ACS Comb Sci 20(4):197-202 (2018)
Chen PC, Masiewicz P, Rybin V, Svergun D, Hennig J
|
| RgGuinier |
2.0 |
nm |
| Dmax |
6.9 |
nm |
| VolumePorod |
26 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Protein sex-lethal mutant monomer, 20 kDa Drosophila melanogaster protein
|
| Buffer: |
10 mM KP, 50 mM NaCl, 10 mM DTT, pH: 6 |
| Experiment: |
SAXS
data collected at BM29, ESRF on 2017 Feb 2
|
A General Small-Angle X-ray Scattering-Based Screening Protocol Validated for Protein-RNA Interactions.
ACS Comb Sci 20(4):197-202 (2018)
Chen PC, Masiewicz P, Rybin V, Svergun D, Hennig J
|
| RgGuinier |
2.1 |
nm |
| Dmax |
7.4 |
nm |
| VolumePorod |
27 |
nm3 |
|
|
|
|
|
|
|
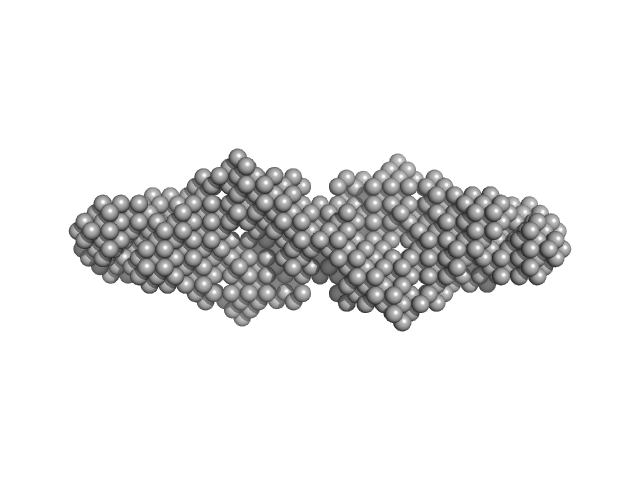
| Sample: |
Hypothetical protein CTHT_0072540 tetramer, 62 kDa Chaetomium thermophilum protein
|
| Buffer: |
20 mM HEPES, 100 mM NaCl, 2 mM β-mercaptoethanol, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Jul 30
|
Prp19/Pso4 Is an Autoinhibited Ubiquitin Ligase Activated by Stepwise Assembly of Three Splicing Factors.
Mol Cell 69(6):979-992.e6 (2018)
de Moura TR, Mozaffari-Jovin S, Szabó CZK, Schmitzová J, Dybkov O, Cretu C, Kachala M, Svergun D, Urlaub H, Lührmann R, Pena V
|
| RgGuinier |
4.1 |
nm |
| Dmax |
16.2 |
nm |
| VolumePorod |
145 |
nm3 |
|
|
|
|
|
|
|
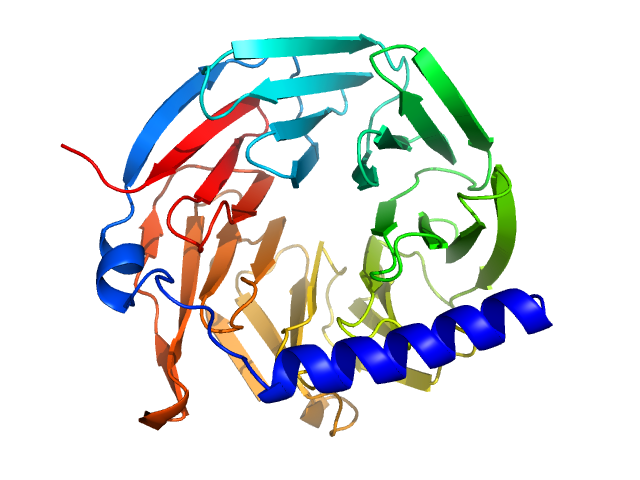
| Sample: |
Hypothetical protein CTHT_0072540 monomer, 35 kDa Chaetomium thermophilum protein
|
| Buffer: |
20 mM HEPES, 100 mM NaCl, 2 mM β-mercaptoethanol, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Jul 19
|
Prp19/Pso4 Is an Autoinhibited Ubiquitin Ligase Activated by Stepwise Assembly of Three Splicing Factors.
Mol Cell 69(6):979-992.e6 (2018)
de Moura TR, Mozaffari-Jovin S, Szabó CZK, Schmitzová J, Dybkov O, Cretu C, Kachala M, Svergun D, Urlaub H, Lührmann R, Pena V
|
| RgGuinier |
2.3 |
nm |
| Dmax |
5.8 |
nm |
| VolumePorod |
68 |
nm3 |
|
|
|
|
|
|
|
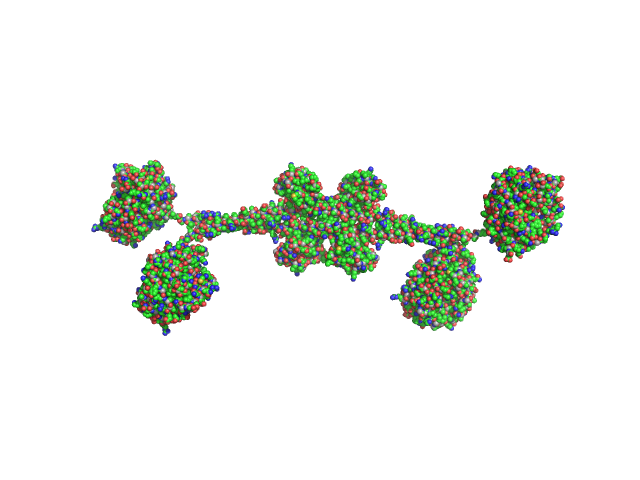
| Sample: |
Full-length hypothetical protein CTHT_0072540 tetramer, 207 kDa Chaetomium thermophilum protein
|
| Buffer: |
20 mM HEPES, 100 mM NaCl, 2 mM β-mercaptoethanol, pH: 7.5 |
| Experiment: |
SAXS
data collected at I911-4, MAX IV on 2013 Oct 15
|
Prp19/Pso4 Is an Autoinhibited Ubiquitin Ligase Activated by Stepwise Assembly of Three Splicing Factors.
Mol Cell 69(6):979-992.e6 (2018)
de Moura TR, Mozaffari-Jovin S, Szabó CZK, Schmitzová J, Dybkov O, Cretu C, Kachala M, Svergun D, Urlaub H, Lührmann R, Pena V
|
| RgGuinier |
6.2 |
nm |
| Dmax |
23.0 |
nm |
| VolumePorod |
280 |
nm3 |
|
|
|
|
|
|
|
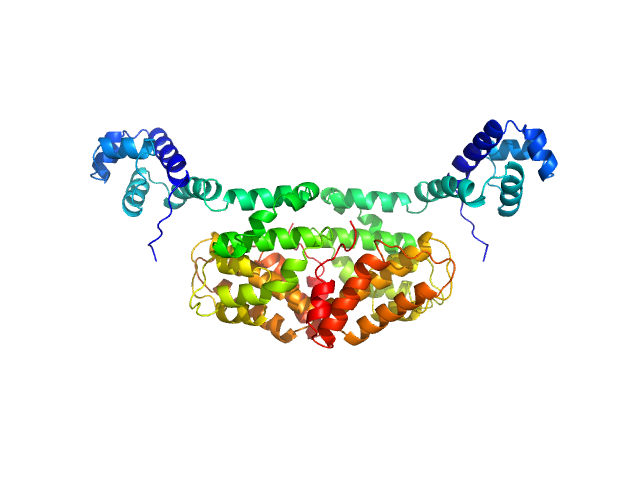
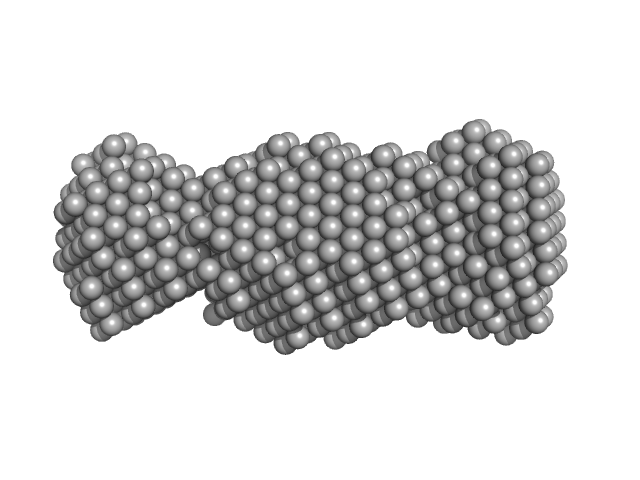
| Sample: |
SaPIbov1 pathogenicity island repressor dimer, 64 kDa Staphylococcus aureus protein
|
| Buffer: |
50 mM HEPES 300 mM NaCl 5 mM MgCl2, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2016 Jul 9
|
Structural model of human dUTPase in complex with a novel proteinaceous inhibitor.
Sci Rep 8(1):4326 (2018)
Nyíri K, Mertens HDT, Tihanyi B, Nagy GN, Kőhegyi B, Matejka J, Harris MJ, Szabó JE, Papp-Kádár V, Németh-Pongrácz V, Ozohanics O, Vékey K, Svergun DI, Borysik AJ, Vértessy BG
|
| RgGuinier |
3.3 |
nm |
| Dmax |
10.5 |
nm |
| VolumePorod |
100 |
nm3 |
|
|
|
|
|
|
|
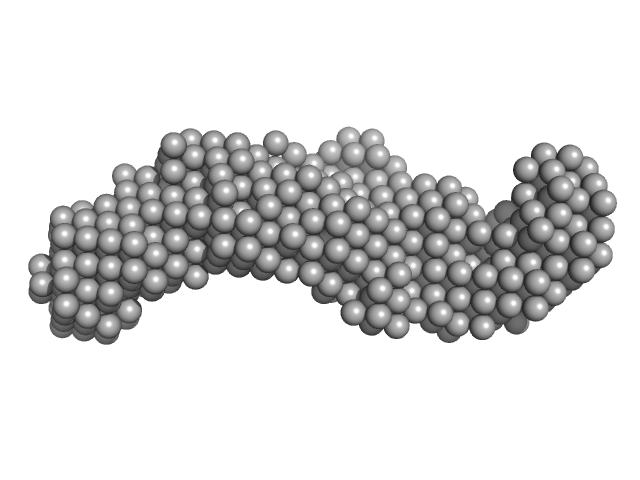
| Sample: |
CS domain protein, putative monomer, 19 kDa Plasmodium falciparum protein
|
| Buffer: |
25 mM Tris-HCl, 100 mM NaCl, 2 mM EDTA, 1 mM B-mercaptoethanol, pH: 7.4 |
| Experiment: |
SAXS
data collected at SAXS2 Beamline, Brazilian Synchrotron Light Laboratory on 2015 May 26
|
Comparative studies of the low-resolution structure of two p23 co-chaperones for Hsp90 identified in Plasmodium falciparum genome.
Int J Biol Macromol 108:193-204 (2018)
Silva NSM, Seraphim TV, Minari K, Barbosa LRS, Borges JC
|
| RgGuinier |
2.5 |
nm |
| Dmax |
8.5 |
nm |
|
|
|
|
|
|
|
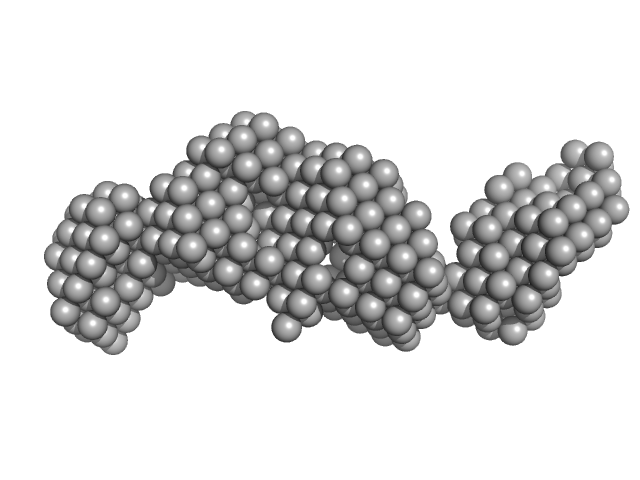
| Sample: |
Co-chaperone p23 monomer, 31 kDa Plasmodium falciparum protein
|
| Buffer: |
25 mM Tris-HCl, 100 mM NaCl, 2 mM EDTA, 1 mM B-mercaptoethanol, pH: 7.4 |
| Experiment: |
SAXS
data collected at SAXS2 Beamline, Brazilian Synchrotron Light Laboratory on 2015 May 26
|
Comparative studies of the low-resolution structure of two p23 co-chaperones for Hsp90 identified in Plasmodium falciparum genome.
Int J Biol Macromol 108:193-204 (2018)
Silva NSM, Seraphim TV, Minari K, Barbosa LRS, Borges JC
|
| RgGuinier |
3.7 |
nm |
| Dmax |
13.0 |
nm |
|
|
|
|
|
|
|
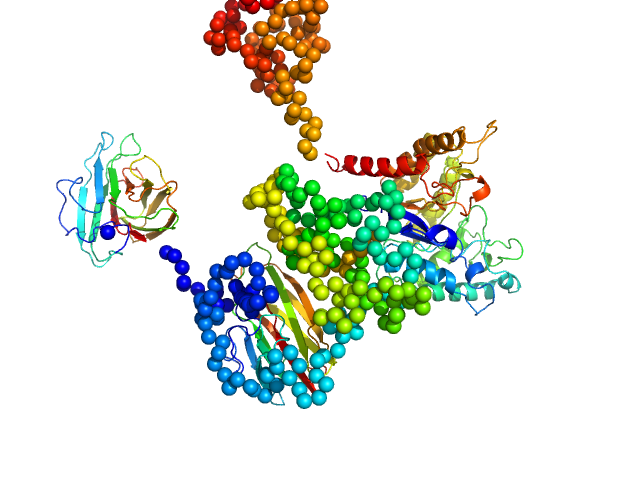
| Sample: |
Methylated C-terminal ZBTB38 binding sequence monomer, 17 kDa DNA
|
| Buffer: |
10 mM Tris, 1 mM tris(2-carboxy-ethyl)phosphine (TCEP), 0.05% NaN3, 10% D2O, pH: 6.8 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSess, Department of Chemistry, University of Utah on 2016 Oct 27
|
The C-Terminal Zinc Fingers of ZBTB38 are Novel Selective Readers of DNA Methylation.
J Mol Biol 430(3):258-271 (2018)
Pozner A, Hudson NO, Trewhella J, Terooatea TW, Miller SA, Buck-Koehntop BA
|
| RgGuinier |
2.5 |
nm |
| Dmax |
9.2 |
nm |
| VolumePorod |
23 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Prebiotic-producing GH10 xylanase (RmXyn10A, full length) monomer, 108 kDa Rhodothermus marinus protein
|
| Buffer: |
50 mM TRIS, 0.5 M NaCl, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2004 Mar 12
|
Structural insights of RmXyn10A - A prebiotic-producing GH10 xylanase with a non-conserved aglycone binding region.
Biochim Biophys Acta Proteins Proteom 1866(2):292-306 (2018)
Aronsson A, Güler F, Petoukhov MV, Crennell SJ, Svergun DI, Linares-Pastén JA, Nordberg Karlsson E
|
| RgGuinier |
3.9 |
nm |
| Dmax |
12.5 |
nm |
| VolumePorod |
170 |
nm3 |
|
|